
Computed tomography of the chest in unilateral pleural effusions: outcome of the British Thoracic Society guideline
Introduction
Identifying the etiology of a unilateral pleural effusion is a clinical challenge. More than 50 different causes have been described, including both localized pleural diseases and systemic conditions (1). The incidence of malignant disease is 20–70% depending on the study population (2-5). According to the guideline by the British Thoracic Society (BTS), initial work-up of a unilateral pleural effusion includes a medical history, physical examination and a review of prescribed and over-the-counter drugs (1). In unsolved cases, the guideline suggests a chest X-ray and aspiration of pleural fluid for cytology and biochemical characterization according to Light’s criteria (1,6) (see Figure 1). If the fluid is not a transudate and fluid analysis and clinical features are not diagnostic, it is recommended to perform a contrast enhanced computed tomography (CT) scan of the thorax.
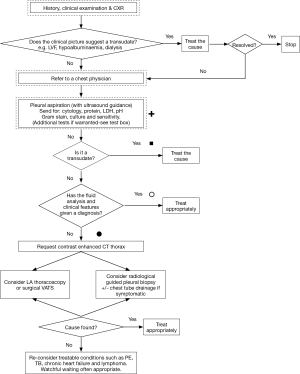
The recommendation of CT is based on five studies (1): one study on empyema (7), two studies on pleural thickening (8,9), one summary article mainly including studies on patients with empyema and pleural thickening (10), and one study including patients with pleural effusions (11).
In addition, it has not previously been investigated how often the guidelines’ recommendation of CT is followed, which is in contrast to what is known about other guidelines (12-14).
On this background, we set out to investigate whether (I) clinicians follow BTS guidelines’ recommendations with respect to CT in patients with unilateral pleural effusions and (II) the diagnostic consequences of following, respectively not following this BTS guidelines.
Methods
Ethics
The study was a retrospective observational study, without randomization or study-specific interventions. Such types of studies are exempt from approval by the local Research Ethics Committee according to Danish legislation (ID 17-000048). The study was approved by the Danish Data Protection Agency (REG-147-2017) and was reported to The Danish Patient Safety Authority.
Study design and participants
In this retrospective cohort study, we identified all adult patients consecutively referred with unilateral pleural effusion to the Department of Pulmonary Medicine at Naestved and Roskilde hospitals (tertiary referral centers), Region Zealand, Denmark, from January 2013 to December 2016. Information on patient demographics, clinical and para-clinical information and investigations were retrieved from the hospital database.
For the purpose of a sub-analysis on the diagnostic value of CT, patients having had both thoracentesis, chest X-ray and CT of the chest performed, but no known malignancy in the thorax, were selected. All patients were followed for a minimum of 12 months.
Outcome of BTS guidelines
According to the diagnostic algorithm in the BTS guideline, pleural fluid biochemical analysis should be performed when patients are referred to a chest physician (see Figure 1).
- We registered in how many cases the pleural fluid was classified as a transudate or exudate according to Light’s criteria (6);
- If the pleural fluid was classified as a transudate, the cause should be treated. We registered in how many patients CT was performed;
- If the pleural fluid was classified as an exudate, two diagnostic approaches exist: if fluid analysis and clinical features has given a diagnosis, then the patients should be treated appropriately. Secondly, if fluid analysis and clinical features has not given a diagnosis, a CT should be performed. Therefore, we registered patients not investigated with a CT and if the physicians’ provisional diagnosis were correct. If a CT was performed, we calculated diagnostic values as stated below.
Classification of the pleural fluid
Light’s criteria (6) were used. The pleural effusion was considered an exudate if one or more of the following criteria were met:
- Pleural lactate dehydrogenase/serum lactate dehydrogenase ratio >0.60;
- Pleural protein/serum protein ratio >0.50;
- Pleural lactate dehydrogenase >2/3 of normal upper limit.
The pleural effusion was considered a transudate if neither of these criteria were met.
CT
All patients underwent contrast-enhanced CT of the chest and upper abdomen. CT was performed using standard protocols: before CT imaging [Philips Brilliance (multi-slice) 64 or iCT 256, Best, The Netherlands], 100 mL Optiray 300 mg I/mL or 100 mL Iomeron 350 mg I/mL was injected intravenously (flow rate 4 mL/s) followed by a bolus of 10 mL isotonic NaCl. All examinations were read by two radiologists and a report was written after having reached consensus (routine procedure).
Classification of results
Cytological examination of the pleural fluid, chest X-ray and CT findings were categorized as below. The investigations were classified blinded to results of later examinations performed and the clinical course.
Cytological examination of pleural fluid
All pleural fluid cytological examinations in Denmark are recorded at The Danish Pathology Register, a national database including data from all pathological examinations in Denmark since 1990 (15). We registered if malignant cells were found during routine examination.
Chest X-ray
The classification of each chest X-ray was based on the routine description as follows:
- X-ray not suspicious for malignancy;
- X-ray suspicious for malignancy:
- If any of the following was found: hilar enlargement or consolidation or atelectasis described as suspicious for malignancy, solitary nodules >20 mm, multiple nodules, any masse(s) single or multiple, and pleural opacities;
- X-ray inconclusive.
Patients with an inconclusive X-ray was included in the analysis of the diagnostic value of CT.
CT results
The classification of each CT-scan was based on the routine scan report as follows:
- CT not suspicious of malignancy;
- CT suspicious of malignancy:
- If any of the following was found: circumferential pleural thickening, nodular pleural thickening, parietal pleural thickening >1 cm or mediastinal pleural involvement and/or showed parenchymal abnormalities (nodules >8 mm) (8,16).
- CT inconclusive:
- None of the above.
An inconclusive CT was classified after a worst-case scenario thus as incoherent with the final diagnosis (17).
The final diagnosis
A final diagnosis of malignancy was based on a multi-disciplinary team decision and tissue biopsies (e.g., transthoracic needle aspiration/biopsy or thoracoscopy).
We searched electronic medical records including The Danish Pathology Register (15) for new diagnoses of malignancy within 12 months after thoracentesis.
A non-malignant, final diagnosis was defined as no pathoanatomical findings of malignancy within one year from thoracentesis.
Statistics
Data were presented as frequencies and/or median and range. Based on a classification of the suggested diagnoses as true-positive (TP), true-negative (TN), false-positive (FP), false-negative (FN), we calculated the sensitivity, specificity, positive likelihood ratio (LR+), negative likelihood ratio (LR−), positive predictive value (PPV), negative predictive value (NPV), and diagnostic accuracy [(TP + TN)/(TP + FP + TN + FN)].
In case of equivocal findings on CT a worst-case scenario was defined as: if the final diagnosis was malignant, the CT was categorized as not suspicious of malignancy and if the final diagnosis was non-malignant, the CT was categorized as suggestive of malignancy.
Categorical data were analyzed using Chi-square test or Fishers Exact test, were appropriate. Mann-Whitney’s test (Wilcoxon rank-sum test) was used for continuous data. Bayesian statistics were used to calculate the post-test probability of malignancy.
Data were analyzed using STATA (StataCorp LLC, version 15.0, College Station, Texas, USA).
Results
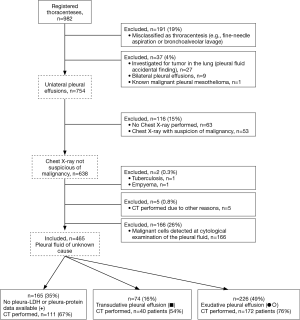
Figure 2 depicts the flow of patients. In total, 465 patients were eligible presenting with a unilateral pleural effusion of unknown cause after baseline examination (chest X-ray, and pleural fluid cytology and culture). Median age was 74 (range, 22–99) years, 167 (36%) were females, and CT was performed in 323 patients (69%).
Adherence to BTS’ guidelines with respect to CT
Measurement of pleural lactate dehydrogenase and/or protein was absenting in 165/465 patients (35%). Of these, 111 patients underwent CT (67%). In 74/465 patients (16%) the pleural fluid was classified as a transudate regardless hereof, 40 patients (54%) underwent a CT.
The remaining 226/465 patients (49%) were classified as having an exudate, which in 23 patients was diagnosed as secondary to specific, non-malignant diseases (most often congestive heart failure or renal failure). CT was performed in 172 of the remaining 203 patients (85%).
Of the 323 patients who underwent CT, the referral was in accordance with BTS guidelines in 172 patients (53%) (Figure 1) (1).
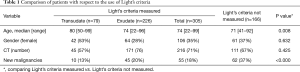
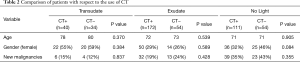
Patients in whom Light’s criteria were not applied, were significantly younger and more often diagnosed with malignancy, but we found no difference in gender and the use of CT (see Table 1). Overall, there was no difference in age, gender and number of malignancies between patients with and without CT (Table 2).
Full table
Full table
Patients not classifiable according to Light’s criteria
Out of 465 patients, 165 had a pleural effusion not classified according to Light’s criteria (see Figures 1 and 2). Forty-five patients (27%) were diagnosed with a new malignancy and 17 patients (10%) were diagnosed with malignant pleural effusion from a previously known primary cancer; in total 62 patients (38%) with: lung cancer (n=36; 58%), malignant pleural mesothelioma (n=8; 13%), breast cancer (n=5; 8%), malignant lymphoma (n=4; 6%), esophagus cancer (n=2; 3%), laryngeal cancer (n=2; 3%) and five patients (8%) with either malignant melanoma, gallbladder cancer, liver cancer, tongue cancer or cancer of unknown primary. Six patients (4%) were lost to follow-up.
Pleural transudate not investigated with CT
Following guidelines, 34/465 patients had a pleural transudate and a CT was not performed (see Figures 1 and 2). Of these, two patients (6%) were diagnosed with lung cancer with pleural metastases and two patients (6%), who had previously received intended curative treatment for non-metastatic lung cancer, had recurrence with pleural metastases. One patient (3%) were lost to follow-up.
Pleural transudate investigated with CT
In spite of the guideline recommendations, 40/465 patients with a pleural transudate underwent CT (see Figures 1 and 2). Of these, six patients (15%) were diagnosed with malignancy: lung cancer (n=4; 67%), malignant pleural mesothelioma (n=1; 17%) and bladder cancer (n=1; 17%).
CT was performed due to suspected malignancy in 22/40 patients (55%), including 12 with known, non-pleural malignancy. CT was performed in 10/40 patients (25%) due to suspected non-malignant causes (e.g., empyema, pulmonary embolism).
Four patients (10%) were lost to follow-up.
Pleural exudate investigated with a CT
Following the guideline recommendations, 172/465 patients with a pleural exudate underwent CT (see Figures 1 and 2). Of these, 21 patients (12%) were diagnosed with a new malignancy and 11 patients (6%) were known with a non-pleural malignancy prior to referral, in total 32 patients (19%) with: lung cancer (n=13; 41%), malignant pleural mesothelioma (n=6; 19%), breast cancer (n=6; 19%), malignant lymphoma (n=3; 9%), and 4 patients (13%) with either renal cancer, gastric cancer, tonsillar cancer and thymoma.
Of the remaining 140 patients without a diagnosis of malignancy (81%), 22 (16%) died during follow-up.
Pleural exudate not investigated with a CT
Despite having a pleural exudate, 55/465 patients did not undergo a CT (see Figures 1 and 2). Of these, 11 patients (20%) were diagnosed with a new malignancy, and 2 patients were known with non-pleural malignancy; in total 13 patients (24%) with: lung cancer (n=3; 23%), lung cancer and malignant lymphoma (n=1), malignant pleural mesothelioma (n=3; 23%), ovarian cancer (n=2; 15%) and 4 (31%) with one of the following: breast cancer, kidney cancer, malignant lymphoma and pancreatic cancer.
18F-FDG positron emission tomography (PET)/CT without prior CT was performed in 32/55 patients (58%) and all 13 malignant cases were in this group.
The 23 patients (42%) who were judged to have non-malignant cause of the pleural effusion were managed appropriately and none developed malignancy during the follow-up period. Nine patients (39%) died during follow-up.
Diagnostic value of guideline-based CT (exudative pleural effusions)
A total of 172 patients with exudative pleural effusions underwent a CT. In one patient, the CT was performed at another location and it was not possible to retrieve CT images or scan report, leaving 171 for this analysis. The CT was classified as inconclusive in 10 patients (6%) and they were included as specified under methods. In total, 32/172 patients (19%) were diagnosed with malignancy during the study period.
Overall, CT was suggestive of malignancy in 35 patients (20%), and 15 of these (43%) were diagnosed with malignancy. The sensitivity was 47% (29–65%) and negative-predictive value was 88% (83–91%) (see Table 3).

Full table
In patients with thoracic malignancies, the CT was suggestive of malignancy in 28 patients (18%), and of these, 8 (29%) were diagnosed with malignancy; the sensitivity was 42% (20–67%) and negative predictive value was 92% (88–94%) (see Table 3).
In patients with extrathoracic malignancy, the CT was suggestive of malignancy in 27 patients (18%), and of these, 7 (26%) were diagnosed with malignancy. The sensitivity was 54% (25–81%) and negative-predictive value was 95% (92–97%) (see Table 3).
Diagnostic value of non-guideline-based CT
Overall 151 patients were included in the analysis; transudates n=40 and not classifiable according to Lights criteria n=111. The CT was classified as inconclusive in seven patients (6%) and they were included as specified under methods. In total, 43 patients (28%) were diagnosed with malignancy during the study period.
Overall, The CT was suggestive of malignancy in 52 patients (34%), and of these, 30 (58%) were diagnosed with malignancy. The sensitivity was 70% (54–83%) and negative-predictive value was 87% (81–91%) (see Table 3).
In patients with thoracic malignancies, the CT was suggestive of malignancy in 43 patients (31%), and of these, 21 (49%) were diagnosed with malignancy; sensitivity 68% (49–83%) and negative predictive value 90% (84–94%) (see Table 3).
In patients with extrathoracic malignancy, the CT was suggestive of malignancy in 31 patients (21%), and of these, 9 (29%) were diagnosed with malignancy. The sensitivity was 75% (43–95%) and the negative-predictive value was 97% (91–99%) (see Table 3).
The diagnostic value of guideline versus non-guideline supported CT
The sensitivity of a non-guideline supported CT was significantly higher compared to a guideline supported CT (70% and 47%, respectively), P value <0.045. There was no statistical difference in specificity.
Clinical application
According to the Bayesian method, estimates of the post-test probability of a malignant unilateral pleural effusion in patients who underwent a CT is a function of disease prevalence (pretest probability). The disease prevalence in our population was 25%, which equals other findings in Europe (3,4).
In all patients with CT performed according to the BTS guideline, the findings of a positive CT (LR positive 3.26), would increase this probability to 52% (47–58%), whereas a negative result (LR negative 0.62) would decrease the probability of malignancy to 17% (15–19%).
In all patients with CT not performed in accordance with the BTS guideline, the findings of a positive CT (LR positive 3.42) would increase this probability to 53% (49–57%), whereas a negative result (LR negative 0.38) would decrease the probability of malignancy to 11% (9–13%).
Discussion
This is the first study to investigate if patients with unilateral pleural effusion are investigated with CT according to the BTS guideline (1). We found that in almost half of the patients in our study population (47%), the decision of performing CT was not in agreement with the BTS recommendations (1). E.g., 54% of patients with pleural transudates had undergone CT, which is not recommended by the guideline. Furthermore, the sensitivity of a non-guideline supported CT was significantly higher than a guideline-supported CT.
The sensitivity of CT for predicting malignancy in pleural effusions have been investigated in five studies, yet none reported data on unilateral effusions in isolation (11,18-21).
Our findings are in accordance with three of the five above studies (18,19,21). The sensitivity was higher in two studies (86% and 92%, respectively) (11,20). Patients included in these studies were highly suspicious for malignancy or referred to thoracoscopy, and the incidence of malignancy was higher compared to our study (80% and 68%, respectively) (11,20). These patients may have had a higher level of clinical disease stage, resulting in pathoanatomical changes which can be more easily identified on CT (i.e., lower rate of false negatives). We found an incidence of malignancy of 25%, which is in accordance with previous findings of approximately 20% (3,4).
This difference in study population could also explain the superior specificity found by Traill et al. (100%) (11).
Two studies found a specificity of 93% and 92%, respectively (19,21). In one of the studies, an advanced score was calculated based on logistic regression findings (21). This could result in fewer false positive findings (not specified), and thus a superior specificity (21).
In the last study (21), it is uncertain what caused the difference in specificity, the study population is identical, however, the type of CT used, how the images were analyzed, and how the findings were classified was not stated (19).
Thoracic ultrasound has a high sensitivity for the detection of pleural fluid [100% if >100 mL (22)], and is, in addition, used for image-guided techniques (e.g., tissue biopsies, thoracentesis, and chest tube insertion) (23). Thoracic ultrasound was found to have a sensitivity of 73% and specificity of 100% for predicting malignancy in patients with a pleural effusion, the corresponding numbers for CT were sensitivity 97% and specificity 89% (24). CT correctly identified 32/33 patients with malignant causes compared to 26/33 for thoracic ultrasound (24). However, CT identified two patients as false positive, whereas thoracic ultrasound identified all patients with benign disease (24).
The superior sensitivity of non-guideline supported CT (compared to guideline-supported), could be explained by physicians not measuring pleural LDH or protein in patients with a high suspicion of malignant disease. Several clinical features can predict malignancy in patients with pleural effusions referred to thoracoscopy (20). Alternatively, the differentiation into exudates and transudates do not assist in the decision of performing CT.
The incidence of malignancy in patients with pleural exudates is approximately 30% (4,19) and in pleural transudates 10% (25,26). Because of this, among others, it has been suggested to perform intensive investigations in all patients presenting with a unilateral pleural effusion (19,27).
In the daily clinical work up, the clinicians base the handling of the patients on the descriptions of the CT from the department of radiology. It was not the aim of this study to examine inter- and intra-observer variation among different assessors (17). The aim was solely to investigate what comes from following the BTS guidelines in everyday clinical life.
A strength of the study is, that it is the first study investigating the use of the BTS guidelines’ recommendations in the clinical work up of unilateral pleural effusions, i.e., including unselected, consecutive patients with a unilateral pleural effusion regardless of the presence of pleural abnormalities (e.g., thickening or nodules) and excluding patients suggestive of malignancy at either pre-CT pleural fluid cytology or chest X-ray (11,18,21). Furthermore, our study it is the largest study investigating the value of CT in unilateral pleural effusions and exudates, and the third largest when including studies on pleural lesions and both bilateral and unilateral pleural effusions (18,21).
Overall, approximately two-thirds of the patients were investigated with a CT, independent of the classification into transudates and exudates. In addition, the sensitivity of a non-guideline CT was superior compared to a CT performed in accordance with the BTS guideline. Having in mind that approximately 10% of the patients with a pleural transudate were diagnosed with a malignancy, physicians must consider if a CT should be performed in this setting.
One third of the patients (n=25; 33%) who underwent CT and had a malignant cause, were found to have an extrathoracic malignancy. The guideline recommends a contrast-enhanced chest CT (1), however, future patients might benefit from performing CT of the thorax and abdomen. Future studies need to evaluate, whether this will increase the diagnostic value of CT.
In general, a good diagnostic test provides a LR+ >10 and a LR− <0.1 (28,29). We found a LR+ of 3.42 and LR− of 0.38, which is indeed concerning because it may lead to a high number of superfluous investigations in a substantial number of patients and a considerable risk of missing malignancy in others.
We speculate that the addition of PET-CT would increase the positive predictive value and sensitivity. One retrospective study found a higher sensitivity and equal specificity of FDG PET/CT compared to CT alone in distinguishing benign from malignant pleural effusions (30). On the contrary, a meta-analysis concluded that PET-CT should not be used as a routine examination because PET-CT did not change the probability of malignancy sufficiently (31). Both studies included patients with bilateral pleural effusions, and the meta-analysis also included patients with known thoracic malignancies and pleural lesions.
In our study, malignant pleural mesothelioma, lung cancer and malignant lymphoma were predominant, and PET-CT can rule out malignancy in most solitary pulmonary nodules and pleural lesions due to high sensitivity (32,33).
A limitation of the study is the retrospective design, which unavoidably implies a risk for selection bias.
Conclusions
Clinicians appear not to follow BTS guidelines when deciding to perform chest CT, the relevance of this deviation is supported by the superior sensitivity of CT non-guideline supported CT.
However, overall, CT is associated with low sensitivity and specificity for the diagnosis of thoracic malignancy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Such types of studies are exempt from approval by the local Research Ethics Committee according to Danish legislation (ID 17-000048). The study was approved by the Danish Data Protection Agency (REG-147-2017) and was reported to The Danish Patient Safety Authority.
References
- Hooper C, Lee YC, Maskell N, et al. Investigation of a unilateral pleural effusion in adults: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii4-17. [Crossref] [PubMed]
- Hirsch A, Ruffie P, Nebut M, et al. Pleural effusion: laboratory tests in 300 cases. Thorax 1979;34:106-12. [Crossref] [PubMed]
- Marel M, Zrustova M, Stasny B, et al. The incidence of pleural effusion in a well-defined region. Epidemiologic study in central Bohemia. Chest 1993;104:1486-9. [Crossref] [PubMed]
- Porcel JM, Esquerda A, Vives M, et al. Etiology of pleural effusions: analysis of more than 3,000 consecutive thoracenteses. Arch Bronconeumol 2014;50:161-5. [PubMed]
- Prakash UB, Reiman HM. Comparison of needle biopsy with cytologic analysis for the evaluation of pleural effusion: analysis of 414 cases. Mayo Clin Proc 1985;60:158-64. [Crossref] [PubMed]
- Light RW, Macgregor MI, Luchsinger PC, et al. Pleural effusions: the diagnostic separation of transudates and exudates. Ann Intern Med 1972;77:507-13. [Crossref] [PubMed]
- Waite RJ, Carbonneau RJ, Balikian JP, et al. Parietal pleural changes in empyema: appearances at CT. Radiology 1990;175:145-50. [Crossref] [PubMed]
- Leung AN, Muller NL, Miller RR. CT in differential diagnosis of diffuse pleural disease. AJR Am J Roentgenol 1990;154:487-92. [Crossref] [PubMed]
- Scott EM, Marshall TJ, Flower CD, et al. Diffuse pleural thickening: percutaneous CT-guided cutting needle biopsy. Radiology 1995;194:867-70. [Crossref] [PubMed]
- McLoud TC, Flower CD. Imaging the pleura: Sonography, CT, and MR imaging. American Journal of Roentgenology 1991;156:1145-53. [Crossref] [PubMed]
- Traill ZC, Davies RJ, Gleeson FV. Thoracic computed tomography in patients with suspected malignant pleural effusions. Clin Radiol 2001;56:193-6. [Crossref] [PubMed]
- Cabana MD, Rand CS, Powe NR, et al. Why don't physicians follow clinical practice guidelines? A framework for improvement. Jama 1999;282:1458-65. [Crossref] [PubMed]
- Pimlott NJ, Persaud M, Drummond N, et al. Family physicians and dementia in Canada: Part 1. Clinical practice guidelines: awareness, attitudes, and opinions. Can Fam Physician 2009;55:506-7.e1-5.
- Saini SD, Nayak RS, Kuhn L, et al. Why don't gastroenterologists follow colon polyp surveillance guidelines?: results of a national survey. J Clin Gastroenterol 2009;43:554-8. [Crossref] [PubMed]
- Bjerregaard B, Larsen OB. The Danish Pathology Register. Scand J Public Health 2011;39:72-4. [Crossref] [PubMed]
- MacMahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology 2005;237:395-400. [Crossref] [PubMed]
- Cohen JF, Korevaar DA, Altman DG, et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open 2016;6:e012799. [Crossref] [PubMed]
- Hallifax RJ, Haris M, Corcoran JP, et al. Role of CT in assessing pleural malignancy prior to thoracoscopy. Thorax 2015;70:192-3. [Crossref] [PubMed]
- Bintcliffe OJ, Hooper CE, Rider IJ, et al. Unilateral Pleural Effusions with More Than One Apparent Etiology. A Prospective Observational Study. Ann Am Thorac Soc 2016;13:1050-6. [Crossref] [PubMed]
- Ferrer J, Roldan J, Teixidor J, et al. Predictors of pleural malignancy in patients with pleural effusion undergoing thoracoscopy. Chest 2005;127:1017-22. [Crossref] [PubMed]
- Porcel JM, Pardina M, Bielsa S, et al. Derivation and validation of a CT scan scoring system for discriminating malignant from benign pleural effusions. Chest 2015;147:513-9. [Crossref] [PubMed]
- Kalokairinou-Motogna M, Maratou K, Paianid I, et al. Application of color Doppler ultrasound in the study of small pleural effusion. Med Ultrason 2010;12:12-6. [PubMed]
- Havelock T, Teoh R, Laws D, et al. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65 Suppl 2:ii61-76. [Crossref] [PubMed]
- Qureshi NR, Rahman NM, Gleeson FV. Thoracic ultrasound in the diagnosis of malignant pleural effusion. Thorax 2009;64:139-43. [Crossref] [PubMed]
- Moltyaner Y, Miletin MS, Grossman RF. Transudative pleural effusions: false reassurance against malignancy. Chest 2000;118:885. [Crossref] [PubMed]
- Gonlugur TE, Gonlugur U. Transudates in malignancy: still a role for pleural fluid. Ann Acad Med Singapore 2008;37:760-3. [PubMed]
- Walker S, Maskell N. Identification and management of pleural effusions of multiple aetiologies. Curr Opin Pulm Med 2017;23:339-45. [Crossref] [PubMed]
- Hayden SR, Brown MD. Likelihood ratio: A powerful tool for incorporating the results of a diagnostic test into clinical decisionmaking. Ann Emerg Med 1999;33:575-80. [Crossref] [PubMed]
- Šimundić AM. Measures of Diagnostic Accuracy: Basic Definitions. EJIFCC 2009;19:203-11. [PubMed]
- Sun Y, Yu H, Ma J, et al. The Role of 18F-FDG PET/CT Integrated Imaging in Distinguishing Malignant from Benign Pleural Effusion. PLoS One 2016;11:e0161764. [Crossref] [PubMed]
- Porcel JM, Hernandez P, Martinez-Alonso M, et al. Accuracy of fluorodeoxyglucose-PET imaging for differentiating benign from malignant pleural effusions: a meta-analysis. Chest 2015;147:502-12. [Crossref] [PubMed]
- Treglia G, Sadeghi R, Annunziata S, et al. Diagnostic accuracy of 18F-FDG-PET and PET/CT in the differential diagnosis between malignant and benign pleural lesions: a systematic review and meta-analysis. Acad Radiol 2014;21:11-20. [Crossref] [PubMed]
- Madsen PH, Holdgaard PC, Christensen JB, et al. Clinical utility of F-18 FDG PET-CT in the initial evaluation of lung cancer. Eur J Nucl Med Mol Imaging 2016;43:2084-97. [Crossref] [PubMed]


