VATS biopsy for undetermined interstitial lung disease under non-general anesthesia: comparison between uniportal approach under intercostal block vs. three-ports in epidural anesthesia
Introduction
Despite the advent of high-resolution computed tomography (HRCT) or fine needle biopsy surgical lung biopsy is still considered the gold standard to achieve a definitive diagnosis in undetermined interstitial lung disease (ILD) in order to establish a correct therapy and to predict a reliable prognosis (1-3). These procedures have successfully been accomplished via video-assisted thoracic surgery (VATS) with a lower morbidity, less pain and a shorter hospital stay (4-7) in comparison with traditional open accesses. However, one of the major risks during surgical biopsy is still represented by the general anesthesia under one-lung ventilation, which may precipitate altered respiratory function, pulmonary hypertension and infections (8,9). Current guidelines by the American Thoracic Society/European Respiratory Society (10,11) recommend surgical biopsy only for those ILD patients who are at acceptable risk to tolerate the procedure and this has severely limited the use of this precious diagnostic device.
To avoid potential complications we initially introduced awake VATS biopsy under thoracic epidural anesthesia (TEA), but more recently we established a new procedure through a unique access under a simple intercostal block in non-general anesthesia (12). Hereby we analyzed comparative merits of this procedure in comparison to VATS biopsy under TEA.
Methods
This investigation is a branch of the mainstay program started in 2001, previously referred to as “awake thoracic surgery” and now more widely defined as “thoracic surgery under monitored anesthesia care” (13).
Patients
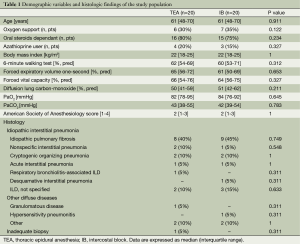
From January 2002 onwards a total of 40 consecutive patients with an undetermined ILD underwent VATS biopsy under non-general anesthesia. In the first 20 patients the VATS biopsy was performed under TEA and in the last 20 with intercostal block through a unique access. Demographics variables and histologic findings are summarized in Table 1. Patients were selected according to clinical and radiologic findings by a multidisciplinary panel formed by one pulmonologist, one thoracic surgeon, one anesthesiologist experienced in non-intubated procedures and one radiologist dedicated to ILD. Patients routinely underwent HRCT (General Electric Medical Systems, Milwaukee, WI, USA) and evaluation included the pattern of parenchymal abnormality (i.e., consolidation, ground-glass opacity, reticular pattern), anatomic distribution and presence of associated pathologies (i.e., mediastinal lymphadenopathy).
Full table
Imaging of diffuse honeycombing pattern was considered by itself a criteria for excluding lung biopsy. Other exclusion criteria were age >75 years, end-stage disease with need of mechanical ventilation, BMI >22 and <30, diffusing lung capacity for carbon monoxide (DLCO) <30% predicted, basal room air PaO2 <50 mmHg, PaCO2 >50 mmHg, American Society of Anesthesiology score <3 and presence of immunodeficiency status or active cancer. We considered tenacious pleura-pulmonary adhesions or patient’s anxiety common contraindication for a non-general anesthesia procedure.
All patients gave their written informed consent. Local institutional review board approval was obtained for study (ref #CT0013-7268). Respiratory assessments included timed spirometry and plethysmography with single-breath DLCO (Vmax 22; Sensor Medics, Yorba Linda, CA, USA) and arterial blood gas analysis. Exercise tolerance was assessed with the standard 6-minute walk test. Quality of life was assessed with the St. George Respiratory Questionnaire (SGRQ) general score (best =0, worst =100) (14,15). In addition all patients underwent preoperative fiberoptic bronchoscopy with bronchoalveolar lavage and cardiac evaluation including color Doppler echocardiography for pulmonary artery pressure non-invasive estimation. Right heart catheterism was performed in selected cases only. Laboratory tests entailed complete blood cell count with differential leukocyte counts, renal and liver function tests, and urinalysis.
Technique
Lung areas suitable for biopsy were chosen after panel discussion. We preferentially chose the middle lobe and the lingula, which are the lung areas most suited to surgical biopsy. The other most targeted areas were the apical segment of the lower lobe or the ventral of the right upper lobe. All these regions easily provided a large quantity of tissue, with a relatively short and straight suture line. The ultimate decision of the area targeted for biopsy was taken intraoperatively according to the most diagnostic site and the most reachable area in a breathing lung. Generally, target areas appeared as cobblestone road with subpleural nodularity, covered by thick and greyish visceral pleura with evidence of neoangiogenesis. These visible findings were usually coupled by the palpatory sensation of an anelastic parenchyma with increased resistance.
A resection volume greater than 1 mL (1 cm3) was usually considered satisfactory and we routinely collected two or more biopsies without creating supplementary incisions.
Patients were continuously monitored by electrocardiogram, pulse oxymeter, systemic and central venous blood pressure, body temperature, arterial blood gases, and end-tidal CO2 by insertion of one detector into a nostril. Forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) were re-assessed immediately pre and postoperatively by a portable spirometer (Vmax Encore 29, Sensor Medics, Yorba Linda, CA, USA).
Intraoperative monitoring included assessment of all these parameters at different standardized times: before incision, at pneumothorax induction, at chest closure and 1-hour postoperatively. Evaluation of acute pain was assessed using a visual analogue scale (VAS) (0= absent, to 10= most severe imaginable pain) (16). Procedures took place in a calm and cooperative setting with a low-volume classical or melodic music played in the background. During the procedure, a venturi mask was used to keep oxygen saturation greater than 90%. Hypercapnia was well tolerated and correction was performed only when PH decreased to less than 7.2.
A chest drain with an underwater seal or a simple endoscopic suction device system was always kept ready to rapidly contrast a discomfort secondary to iatrogenic pneumothorax. Mild sedation with midazolam or propofol was useful to control the discomfort induced by pneumothorax or panic attacks. Shift to general anesthesia was allowed only in the following conditions: irritation or intolerance of the patient before the accomplishment of the biopsy, elevated level of PaCO2 (50 mmHg), operation technically difficult or hemorrhagic complications.
VATS biopsy under TEA
After insertion of venous and radial artery catheters, an epidural catheter was positioned at T4-T5 level through which a bolus of 5 mg ropivacaine plus 5 µg sufentanyl was injected. Continuous infusion of ropivacaine 2 mg/mL (5 mL/hour) was then started 20 minutes prior to operation with the patient lying on the side targeted for biopsy. After achievement of satisfactory anesthesia the patient was turned on the other side ready for the operation.
The procedure was classically carried out through three ports. Usually the camera port was placed in the eighth intercostal space along the midaxillary line, the operating ports were usually placed in the fifth intercostal space, anterior and posterior axillary lines. In the case of biopsies located in the posterior segments of the lower lobes, theoperative port was set more caudally than the camera port and the monitor was positioned at the bottom of the patient to avoid mirror image effect. The biopsy was preferentially performed along a straight line an endostapler endopath® 30 or 45 with staples of 4.5 mm suturing with as less number of bites as possible in order to decrease the risk of bleeding. Blood and air leakage from the resection line were carefully controlled both at the time of suture completion and at chest closure. A 28 CH chest tube was inserted through the lowest incision. At end-procedure, lung re-expansion was achieved under thoracoscopic vision by asking the patient to breathe deeply and cough repeatedly. The epidural catheter was usually removed on postoperative day one.
Uniport VATS biopsy under intercostal block
After insertion of venous and radial artery catheters, an aerosolized 5 mL solution of 2% lidocaine was administered for 5 minutes in order to avoid cough reflex. The intercostal block was accomplished by local injection of 20-30 mL solution of 2% lidocaine and 7.5% ropivacaine, for achieving a rapid onset with a long duration of the analgesic effect. Site of inoculation was done along the space selected for uniportal VATS and included subcutaneous layers, intercostal nerves and parietal pleura. The grade of local anesthesia was always adequate. In a few cases benzodiazepine (midazolam 0.03-0.1 mg/kg) or opioids (remifentanil 15 µg/kg/min) were intravenously supplemented during lung manipulation or stapling manoeuvres.
All VATS biopsies were carried out from a single small 30-40 mm uniport skin incision carried out along the space judged the most suitable to reach the foreseen area. In the case of lingula or middle lobe biopsy incision was usually performed along the fourth intercostal space medially from the anterior axillary line, whereas posterior segments were biopsied through an eighth intercostal space posterior incisions. Rib spreading by retractor was always avoided. Through the incision we introduced the operative thoracoscope, the articulated stapler and incidentally a gauze pad mounted on a ring-forceps in order to contrast lung movements during breathing or coughing. In many instances we were able to exteriorize the most distal target area and accomplish the resection outside from the chest. At the end of the procedure one 28 CH chest tube was collocated through the posterior end of the incision. No trans-intercostal suture was necessary. Muscle sutures were tightened after asking the patient to breathe deeply or cough to achieve maximal lung re-expansion.
Postoperative care
Postoperative care was similar after both procedures. After a short stage in the weaning areas the patient was directly sent to the ward. Liquids infusion was stopped immediately and drinking, meal intake and ambulation were started on the same day of surgery. Chest X-ray was routinely performed at 24 hours from the procedure to confirm adequate lung expansion.
State of consciousness and postoperative recovery was evaluated by the quality of recovery (QoR-40), which is a 40-item self-administered questionnaire (17). Each item is linked to a 5-point Likert scale [1-5] with a minimum cumulative score of 5 (maximal impairment) and maximum of 200 (no impairment). Time of discharge was determined by chest tube removal, which generally took place in the absence of air leak and with a daily fluid leakage less than 150 mL.
The biopsy samples were sent fresh and reviewed by the institutional pathologist with a hub on ILD. A fragment was also sent for microorganism cultures.
Statistics
All data was statistically analyzed using the SPSS (SPSS® 9.05 for Windows, SPSS Inc., Chicago, 1998). Interdependence among factors and group comparisons were prudentially assessed by non-parametric tests. Data were expressed with median and interquartile range deviation. P values <0.05 were regarded as statistically significant in two tailed tests.
Results
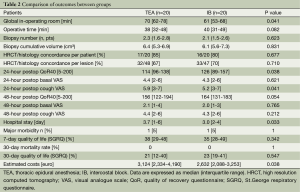
Two patients, one from each group, required the shift to general anesthesia with intubation and single lung ventilation. No patient needed conversion to open thoracotomy.
A total of 95 biopsies were performed: 48 (2.4 per patient) in TEA group and 47 (2.35 per patient) in intercostal block group, respectively. No 30-day postoperative mortality was experienced. We reported one case of acute respiratory insufficiency resolved by non-invasive ventilation in the TEA group and one acute pneumonia in the intercostal block group. We also recorded minor complications related to TEA: hypotension antagonized by noradrenalin infusion (n=2) and urinary block (n=3) requiring catheterization.
Global operative time was significantly shorter for operations performed under intercostal block {61 [53-68] vs. 70 [62-78]; P=0.041} and this was mainly due to the lack of epidural catheter introduction and time for the onset of anesthesia.
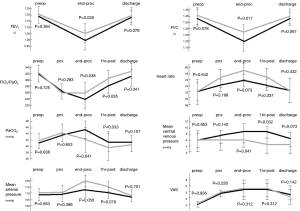
Intraoperative findings of main physiologic parameters are shown in Figure 1. FEV1 and FVC presented a significant decrement in both groups at end-operation, but the fall was lesser in the intercostal one, with a significant difference at intergroup analysis (FEV1, P=0.026 and FVC, P=0.017). As a result, oxygenation (FiO2/PaO2) and PaCO2 were significantly different between groups at end-operation (P=0.038 and P=0.041, respectively) and at 1 hour postoperatively (P=0.035 and P=0.033, respectively). Interestingly, the values of central venous pressure were significantly less elevated during intercostal block at the end of the procedure (P=0.041) and after 1 hour from the procedure (P=0.032). Pain coverage was satisfactory throughout the procedure and without significant differences in both groups (Figure 1). Similarly, there was no difference in basal and under cough VAS at 24 and 48 hours, respectively. Postoperative QoR was significantly better in intercostal group after 24 (P=0.038) hours from the operation, whereas quality of life at 7 and 30 days was similar between groups. Hospital stay was significantly shorter in patients undergoing intercostal block (P=0.033) and this also impacted the economic expenses (P=0.038) (Table 2).
Full table
A reliable pathologic specimen was obtained by surgical biopsy 97.5% (39/40) of the patients, and in 94.7% (90/95) of biopsies. The biopsy specimens were concordant in 82.5% (33/40) of patients and in 68.4% (65/95) of the biopsies. No differences were found between groups (Table 2). On the basis of histopathology findings, therapy was adjusted or modified in 21 patients (52.5%).
Discussion
The use of intercostal block associated with intravenous sedation during thoracoscopy for diagnostic and therapeutic purposes has already been described in different thoracic pathologies with satisfactory results (18-21). In this personal series we presented the additional advantages of intercostal block compared to TEA during VATS lung biopsy in patients with ILD undetermined lesions.
In our study we experienced that intercostal block may significantly attenuate intraoperative lung-volumes fall as well as hypoxia (FiO2/PaO2) and hypercapnia respect to same procedures under TEA.
It has been reported that TEA causes a significant decrement of inspiratory capacity (22) as a probable blockade of efferent or afferent pathways of the intercostal nerve roots resulting in a decreased contribution of the rib cage to tidal breathing (23). TEA may also produce a wide adrenergic tone fall with prevalence of bronchus-constriction as evidenced by FEV1 decrement (24). The documented increment of the central venous pressure in TEA could be a consequence of lung flow reduction as well as of the impairment of ventricle sympathetic outflow due to the adrenergic block (25-27).
These effects are absent or minimal under intercostal block, which leaves normal adrenergic tone without interfering on skeletal, bronchus and cardiac muscle motility (12) also avoiding the intrinsic risks and unpleasant side-effects related to TEA.
The risk of coughing is high when stapling the lung under intercostal block but it can be minimized by the use of aerosolized 2% lidocaine with transitory side effects. As far as the expected lesser duration of pain coverage is concerned, we found it more useful to have pain control with intravenous drugs rather than the prolonged and fastidious disturbances following TEA. Furthermore, the QoR-40 questionnaire clearly documented a significant greater and faster postoperative recovery after the intercostal block.
This study is retrospective and non-randomized and the results need to be interpreted as such. Another potential limitation is represented by the reliability of respiratory functional tests performed during a surgical operation, which can be altered by patient fatigue, lack of cooperation and iatrogenic pneumothorax. However these potential artifacts were homogeneous between groups and do not appear to interfere with the global trend of the measurements.
Conclusions
We would suggest that uniportal VATS biopsies under intercostal block can provide better intraoperative and postoperative outcomes compared to TEA, thus increasing the safety as well as enlarging the indication of VATS biopsy in presently not-eligible patients. These results stimulate our interest in thoracic surgery under monitored anesthesia care (12), which permits a quicker postoperative recovery as well as lower morbidity, hospital stay and economical costs with satisfaction of patients, surgeons, pneumologists and administrators.
Acknowledgements
We express our sincere gratitude to all pneumologists, physicians and general practitioners for referring us their patients in order to achieve a definitive diagnosis. Both authors take responsibility for the content of the manuscript, including the data and analysis.
Both authors made substantial contributions to conception, design, acquisition or analysis and interpretation of data; Vincenzo Ambrogi has drafted the submitted article; Tommaso Claudio Mineo has provided final approval of the version to be published; each author has agreed for all aspects of the work in ensuring that any part of the work is appropriately investigated and resolved.
Disclosure: The authors declare no conflict of interest.
References
- Raghu G, Mageto YN, Lockhart D, et al. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest 1999;116:1168-74. [PubMed]
- Flaherty KR, Travis WD, Colby TV, et al. Histopathologic variability in usual and nonspecific interstitial pneumonias. Am J Respir Crit Care Med 2001;164:1722-7. [PubMed]
- Kayatta MO, Ahmed S, Hammel JA, et al. Surgical biopsy of suspected interstitial lung disease is superior to radiographic diagnosis. Ann Thorac Surg 2013;96:399-401. [PubMed]
- Boutin C, Viallat JR, Cargnino P, et al. Thoracoscopic lung biopsy. Experimental and clinical preliminary study. Chest 1982;82:44-8. [PubMed]
- Bensard DD, McIntyre RC Jr, Waring BJ, et al. Comparison of video thoracoscopic lung biopsy to open lung biopsy in interstitial lung disease. Chest 1993;103:765-70. [PubMed]
- Ferson PF, Landreneau RJ, Dowling RD, et al. Comparison of open versus thoracoscopic lung biopsy for diffuse infiltrative pulmonary disease. J Thorac Cardiovasc Surg 1993;106:194-9. [PubMed]
- Krasna MJ, White CS, Aisner SC, et al. The role of thoracoscopy in the diagnosis of interstitial lung disease. Ann Thorac Surg 1995;59:348-51. [PubMed]
- Fishbein MC. Diagnosis: To biopsy or not to biopsy: assessing the role of surgical lung biopsy in the diagnosis of idiopathic pulmonary fibrosis. Chest 2005;128:520S-525S. [PubMed]
- Lettieri CJ, Veerappan GR, Helman DL, et al. Outcomes and safety of surgical lung biopsy for interstitial lung disease. Chest 2005;127:1600-5. [PubMed]
- American thoracic society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 2000;161:646-64. [PubMed]
- American Thoracic Society, European Respiratory Society. American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277-304. [PubMed]
- Mineo TC, Ambrogi V. Efficacy of awake thoracic surgery. J Thorac Cardiovasc Surg 2012;143:249-50. [PubMed]
- Mineo TC, Tacconi F. From “Awake” to “Monitored Anesthesia Care” Thoracic Surgery: a 15 Years Evolution. Thoracic Cancer 2014;5:1-13.
- Jones PW, Quirk FH, Baveystock CM, et al. A self-complete measure of health status for chronic airflow limitation: The S.George’s respiratory disease questionnaire. Am Rev Resp Dis 1992;145:1321-7. [PubMed]
- Carone M, Bertolotti G, Anchisi F, et al. Il Saint George’s Respiratory Questionnaire (SGRQ): la versione italiana. Rass Mal App Resp 1999;14:31-37.
- Price DD, McGrath PA, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain 1983;17:45-56. [PubMed]
- Myles PS, Weitkamp B, Jones K, et al. Validity and reliability of a postoperative quality of recovery score: the QoR-40. Br J Anaesth 2000;84:11-5. [PubMed]
- Rusch VW, Mountain C. Thoracoscopy under regional anesthesia for the diagnosis and management of pleural disease. Am J Surg 1987;154:274-8. [PubMed]
- Danby CA, Adebonojo SA, Moritz DM. Video-assisted talc pleurodesis for malignant pleural effusion utilizing local anesthesia and IV sedation. Chest 1998;113:739-42. [PubMed]
- Nezu K, Kushibe K, Tojo T, et al. Thoracoscopic wedge resection of blebs under local anesthesia with sedation for treatment of a spontaneous pneumothorax. Chest 1997;111:230-5. [PubMed]
- Migliore M, Giuliano R, Aziz T, et al. Four-step local anesthesia and sedation for thoracoscopic diagnosis and management of pleural diseases. Chest 2002;121:2032-5. [PubMed]
- Warner DO, Warner MA, Ritman EL. Human chest wall function during epidural anesthesia. Anesthesiology 1996;85:761-73. [PubMed]
- Kochi T, Sako S, Nishino T, et al. Effect of high thoracic extradural anaesthesia on ventilatory response to hypercapnia in normal volunteers. Br J Anaesth 1989;62:362-7. [PubMed]
- Clemente A, Carli F. The physiological effects of thoracic epidural anesthesia and analgesia on the cardiovascular, respiratory and gastrointestinal systems. Minerva Anestesiol 2008;74:549-63. [PubMed]
- Sundberg A, Wattwil M, Arvill A. Respiratory effects of high thoracic epidural anaesthesia. Acta Anaesthesiol Scand 1986;30:215-7. [PubMed]
- Takasaki M, Takahashi T. Respiratory function during cervical and thoracic extradural analgesia in patients with normal lungs. Br J Anaesth 1980;52:1271-6. [PubMed]
- Sakura S, Saito Y, Kosaka Y. The effects of epidural anesthesia on ventilatory response to hypercapnia and hypoxia in elderly patients. Anesth Analg 1996;82:306-11. [PubMed]


