
ERAS and patient reported outcomes in thoracic surgery: a review of current data
Introduction
Both thoracic and non-thoracic surgeons alike are showing increased interest in enhanced recovery after surgery (ERAS) pathways. These protocolized, evidence-based standards for perioperative patient care have been shown to effectively shorten hospital length of stay and decrease postoperative complication rates (1). As a result, ERAS pathways are becoming more standardized and accepted in the surgical realm. Furthermore, improved surgical outcomes due to ERAS should, in theory, improve our patients’ quality of life.
As a community of thoracic surgeons, our research often focuses on patient survival, perioperative mortality, and complication rates following interventions for specific disease processes. These endpoints are objective and relatively easy to measure using patient medical records, institutional and national outcomes databases. However, patients undergoing major thoracic operations experience a myriad of symptoms that are usually not captured and thus not contained in data analysis. Some symptoms are nonspecific—pain, fatigue, emotional distress, anxiety; others are disease or organ specific—dyspnea, dysphagia, gastrointestinal cramping. Regardless, all have some degree of subjectivity and can be patient-specific. These health-related quality of life (HR-QOL) concerns are increasingly of greater concern to patients and providers alike. The most accurate way to evaluate and measure these symptoms is by gathering this data directly from the patient, without interpretation by medical providers. Such data is typically referred to as patient-reported outcomes (PROs).
In order to improve surgical outcomes and deliver patient-centered care, it is imperative that clinicians start reviewing objective metrics contained within morbidity and mortality data alongside subjective data regarding patients’ experience. This article reviews the current data surrounding both ERAS and PROs within thoracic surgery and investigates how the two concepts are ultimately related. Current challenges, recommendations and guidelines are summarized.
ERAS
Background of ERAS
First described in 2001, ERAS was developed by a group of European surgeons who wished to emphasize that the key endpoint in surgical recovery is quality, not speed (2). Fundamental ERAS components include:
- a multidisciplinary team;
- a multimodal approach to resolving issues that delay recovery and cause complications;
- scientific, evidence-based protocols and;
- changes in patient management using interactive and continuous audit.
The concept of enhanced recovery encompasses the entire patient journey from the time of surgical referral until postoperative discharge from the hospital. Initial ERAS studies across multiple surgical subspecialties report improved patient outcomes and decreased healthcare costs (3-6).
ERAS in thoracic surgery: current data
Over the past decade, multiple studies have shown that ERAS protocols in thoracic surgery decrease the incidence of cardiac and pulmonary complications, reduce opiate usage, minimize fluid overload, shorten length of stay, and decrease hospital costs (7-15). Madani and colleagues report a series of 234 patients undergoing open lobectomy for cancer and conclude that ERAS reduced complication rates from 50% to 37% with no difference in readmission rates or emergency room visits (11). Furthermore, they note that earlier removal of chest tubes and foley catheters shortened length of stay. In another study of 2,886 patients undergoing both open and minimally invasive (VATS) pulmonary resections, Van Haren et al. conclude that ERAS led to a decreased length of stay by one day, decreased pulmonary complications from 29% to 20%, and decreased cardiac complications from 18% to 12% (14). Interestingly, the authors conclude that while ERAS has clear benefit in thoracotomy patients, the study did not show clear benefit of ERAS in patients undergoing minimally invasive surgery. Similarly, in a study investigating ERAS in 600 patients after VATS lobectomy or segmentectomy, Brunelli et al. also conclude that ERAS did not significantly improve measured outcomes (8). The authors believe that many of the ERAS elements were already part of their standard care following VATS, and thus their new protocol may not have been significantly different enough to impact outcomes.
Another study investigating VATS-specific and thoracotomy-specific ERAS protocols, Martin et al. conclude that ERAS shortened length of stay by 2 days in patients undergoing thoracotomy and significantly reduced opiate usage (12). However, similar to the previously mentioned studies no LOS difference was identified after VATS. There were no differences in complication, readmission or mortality rates. Most notably, however, was the finding that ERAS contributed to a cost savings of $5,300 per VATS patient and $15,000 per thoracotomy patient. Finally, a recent prospective study by Rogers et al. examined overall compliance with ERAS pathways, as well as with fifteen individual components of the pathway (7). Univariate analysis revealed that compliance with early mobilization and carbohydrate loading was significantly associated with decreased mortality and shorter length of stay. Multivariate analysis showed that compliance with the entire fifteen-element ERAS pathway was independently associated with decreased mortality.
ERAS guidelines
In summary, standardized ERAS protocols have been shown to reduce cost, complications, and length of stay, without sacrificing quality of care. This is particularly true after thoracotomy, though the impact may not be as great after VATS. Given the abundance of evidence supporting improved outcomes using ERAS protocols in thoracic surgery patients, the ERAS Society and European Society of Thoracic Surgeons (ESTS) recently reviewed 45 ERAS items spanning from initial presentation to postoperative discharge following lung surgery (16). After extensive literature review, the authors graded quality of evidence and consensus recommendations were formed on each topic. A summary of their guidelines can be found in Table 1.
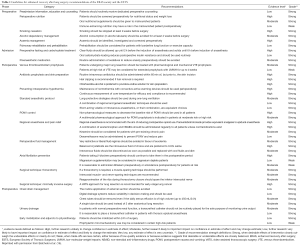
Full table
Patient reported outcomes (PRO)
Background of PRO
In 2013, Basch and colleagues introduced the concept of patient reported outcomes (PRO) and defined them as measures of patient physical and psychosocial well-being obtained by direct patient self-report (Table 2). They may provide a more reliable means of evaluating and comparing postoperative outcomes and effectiveness of various treatment options (17). Furthermore, because PRO measure those outcomes that matter most to patients, they serve as the basis for improved patient-centered care and a reliable means for measuring HR-QOL. As a result, there has been a rapidly increasing demand for the integration of PRO into surgical outcomes research. Several national organizations, including the Center for Medicare and Medicaid Services (CMS), National Quality Forum, National Institutes of Health (NIH), National Cancer Institute, the US Food and Drug Administration (FDA) and the American College of Surgeons (ACS) advocate for integration of PRO into the measurement of patient outcomes and assessments of clinical performance (18). The American College of Chest Physicians (ACCP) has included PRO measures as part of their guidelines for lung cancer treatment, recommending the routine use of HR-QOL instruments in clinical care (19). The Center for Medical Technology Policy has advocated for the use of PRO in all prospective, adult oncology clinical effectiveness research studies (20). Perhaps most notable is the Affordable Care Act’s creation of the Patient-Centered Outcomes Research Institute (PCORI), which has provided nearly $2 billion of funding to promote high-quality clinical effectiveness research through the incorporation of PRO (21).
Current tools for PRO
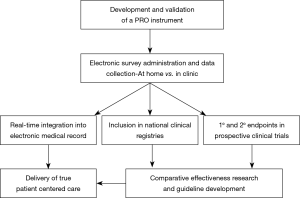
In order to improve upon patient-centered care, PROs must be gathered as part of a routine, standard practice (Figure 1) (22). The ideal tool for data collection must be generalizable, efficient, user-friendly, accurate, and cost-effective. Furthermore, it should integrate into existing clinical workflow and technical infrastructure including the electronic medical record, with minimal burden to the patient and the provider.
Multiple potential PRO instruments exist for use in Thoracic Surgery Patients (Table 3). Our preferred instrument is PROMIS®—a well-validated system of measuring PROs which include a variety of questionnaires that span multiple realms of physical, mental and social health (11,23-27). Because it utilizes a variety of short-form modules across multiple health domains, surveys can be customized to the patient population and disease process of interest. PROMIS questionnaires and instruments use item response theory and computer adaptive testing that acclimates to patient-specific symptoms. Due to its versatility and advantages, it has been recommended by the Center for Medical Technology Policy as one of their preferred PRO measures for cancer clinical research and has been used in a variety of fields including oncology, orthopedics, cardiothoracic surgery, transplantation, and pediatrics (23,26). It is easily translated into a web-based, electronic interface and easily incorporates into several widely-utilized electronic medical record systems.
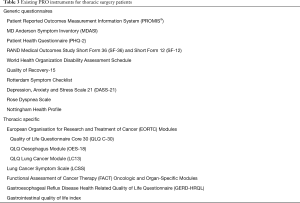
Full table
Other validated and commonly utilized instruments include the following: The MD Anderson Symptom Inventory (MDASI) is generic to all cancers, while the European Organization for Research and Treatment of Cancer Lung Cancer Module (EORC-LC13), Lung Cancer Symptom Scale (LCSS) and Functional Assessment of Cancer Therapy-Lung (FACT-L) are specific to lung cancer (28-31). The Rose Dyspnea Scale specifically measures pulmonary function (scores range 0–4; higher scores indicate worse dyspnea). The Patient Health Questionnaire (PHQ-2), RAND Medical Outcomes Study Short Form (SF-36 and SF-12), and World Health Organization Disability Assessment Schedule (WHODAS) all measure general health (32-34). The Quality of Recovery (QOR-15) measures general health with a short recall period of 24 hours, making it ideal for use in the immediate post-operative period (35).
PRO in thoracic surgery: current data
A variety of both retrospective and prospective studies have examined PRO and HR-QOL results after surgery for thoracic malignancies (27,28,36-43). These studies are relatively small, single center, observational studies. However, valuable information can still be gleaned from them. Several of these studies have compared non-operative vs. operative therapy, VATS vs. thoracotomy, and sublobar resection vs. lobectomy, among other important questions. Overall, these studies show an expected initial decline in physical function, dyspnea, and quality of life scores after surgery, with most studies showing a return to baseline within 6 months to a year.
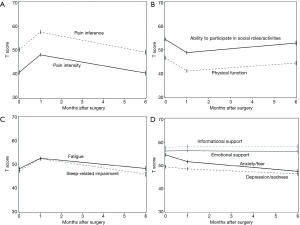
We recently published the initial results of a pilot study investigating the feasibility of integrating PRO into the institutional Society of Thoracic Surgery General Thoracic Surgery Database (STS-GTSD) (27). In this prospective cohort study, 127 patients undergoing lung cancer surgery completed HR-QOL surveys using PROMIS software at their preoperative, initial postoperative and 6-month follow-up clinic appointments. The data were collected electronically on tablet devices and merged with institutional STS data. Similar to other studies, there was a significant increase in pain, fatigue, sleep impairment and decrease in physical function reported at the first postoperative visit. By 6 months, however, these PRO measures generally improved towards baseline (Figure 2, republished with permission). Most importantly, survey completion rates were over 90% and took only 13–15 minutes to complete on average. Since completion of this study, we have further streamlined the survey to require only 3 to 5 minutes to complete at each clinic visit.
Using PRO in ERAS pathways
As discussed earlier, ERAS pathways in thoracic surgery have been shown to improve patient outcomes and decrease hospital costs. Unfortunately, these data alone can paint an incomplete picture of the post-operative experience. The goal of any ERAS pathway is to improve patient recovery after surgery. Improving length of stay and reducing complications is only half of this picture. Improving HR-QOL is the other half, and as discussed PRO are the best way to measure this. In a recent review of ERAS in lung surgery patients, the notion of including PRO is emphasized (44). Eustache et al. highlight the concept of postoperative recovery and “returning to baseline” after surgery. As previously mentioned, most studies have demonstrated a return to functional baseline with 6 months to 1 year of surgery. How patients perceive this recovery process and their HR-QOL along the way may be just as important to them, if not more so, than the objective outcomes physicians often emphasize. As a result, PROs must to be incorporated into ERAS pathways.
Jensen et al. introduced five critical elements of PRO utilization: (I) needs assessment, (II) shared decision-making, (III) symptom management, (IV) outcome assessment, and (V) quality improvement (45). It could be argued, however, that these same concepts parallel the ideology behind ERAS pathways (Table 4). Using a PRO instrument during a patient’s preoperative clinic visit helps assess their clinical needs and can highlight areas of focus for postoperative recovery. Furthermore, because ERAS emphasizes preoperative education and counseling to help patients manage expectations and plan appropriately, gathering baseline QOL metrics helps with shared decision making. During the hospital phase of perioperative recovery, both PRO and ERAS alike focus upon symptom management and outcome assessment and may guide patient specific interventions needed to aide recovery. Lastly, incorporation of PRO into ERAS pathways allows for optimization of quality improvement pathways.

Full table
Finally, a recent article from Refai and colleagues highlights their unique ERAS methodology following thoracic surgery (46). The authors emphasize patient education and counseling through the use of separate patient, surgical, anesthesia, nursing, and respiratory care-plans in the perioperative period. They utilize written material in the form of a booklet that is given to the patient preoperatively. The booklet highlights each of the specific care-pathways and includes a daily checklist so that patients may track their progress following surgery. They also describe the planned development of a digital platform including a smartphone application that would allow for virtual data collection of patient reported outcomes. This would undoubtedly facilitate real-time process improvement in order to optimize patient-centered care.
Conclusions
Quality-focused, cost-effective, patient-centered care is at the forefront of current healthcare reform. Implementation of ERAS pathways in both thoracic and non-thoracic surgery has demonstrated consistent improvement in patient outcomes with an associated decrease in healthcare spending. Furthermore, the incorporation of PRO data into clinical outcomes registries is not only feasible, but also necessary to ensure that the care we deliver meets the needs of patients and stakeholders alike. Without a doubt, clinical practice should adapt recent ERAS guidelines with the goal of on-going quality improvement. Moreover, future studies reporting on surgical outcomes ought to report upon PROs alongside traditional morbidity and mortality data in order to ensure optimal surgical therapy.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Nicholson A, Lowe MC, Parker J, et al. Systematic review and meta-analysis of enhanced recovery programmes in surgical patients. Br J Surg 2014;101:172-88. [Crossref] [PubMed]
- Fearon KC, Ljungqvist O, Von Meyenfeldt M, et al. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr 2005;24:466-77. [Crossref] [PubMed]
- Ljungqvist O, Scott M, Fearon KC. Enhanced Recovery After Surgery: A Review. JAMA Surg 2017;152:292-8. [Crossref] [PubMed]
- Engelman RM, Rousou JA, Flack JE 3rd, et al. Fast-track recovery of the coronary bypass patient. Ann Thorac Surg 1994;58:1742-6. [Crossref] [PubMed]
- Bardram L, Funch-Jensen P, Jensen P, et al. Recovery after laparoscopic colonic surgery with epidural analgesia, and early oral nutrition and mobilisation. Lancet 1995;345:763-4. [Crossref] [PubMed]
- Kehlet H, Mogensen T. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg 1999;86:227-30. [Crossref] [PubMed]
- Rogers LJ, Bleetman D, Messenger DE, et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg 2018;155:1843-52. [Crossref] [PubMed]
- Brunelli A, Thomas C, Dinesh P, et al. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2017;154:2084-90. [PubMed]
- Gimenez-Mila M, Klein AA, Martinez G. Design and implementation of an enhanced recovery program in thoracic surgery. J Thorac Dis 2016;8:S37-45. [PubMed]
- Khandhar SJ, Schatz CL, Collins DT, et al. Thoracic enhanced recovery with ambulation after surgery: a 6-year experience. Eur J Cardiothorac Surg 2018;53:1192-8. [Crossref] [PubMed]
- Madani A, Fiore JF Jr, Wang Y, et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery 2015;158:899-908; discussion 908-10. [Crossref] [PubMed]
- Martin LW, Sarosiek BM, Harrison MA, et al. Implementing a Thoracic Enhanced Recovery Program: Lessons Learned in the First Year. Ann Thorac Surg 2018;105:1597-604. [Crossref] [PubMed]
- Scarci M, Solli P, Bedetti B. Enhanced recovery pathway for thoracic surgery in the UK. J Thorac Dis 2016;8:S78-83. [PubMed]
- Van Haren RM, Mehran RJ, Mena GE, et al. Enhanced Recovery Decreases Pulmonary and Cardiac Complications After Thoracotomy for Lung Cancer. Ann Thorac Surg 2018;106:272-9. [Crossref] [PubMed]
- Semenkovich TR, Hudson JL, Subramanian M, et al. Enhanced Recovery After Surgery (ERAS) in Thoracic Surgery. Semin Thorac Cardiovasc Surg 2018;30:342-9. [PubMed]
- Batchelor TJP, Rasburn NJ, Abdelnour-Berchtold E, et al. Guidelines for enhanced recovery after lung surgery: recommendations of the Enhanced Recovery After Surgery (ERAS(R)) Society and the European Society of Thoracic Surgeons (ESTS). Eur J Cardiothorac Surg 2019;55:91-115. [Crossref] [PubMed]
- Basch E, Torda P, Adams K. Standards for patient-reported outcome-based performance measures. JAMA 2013;310:139-40. [Crossref] [PubMed]
- Lipscomb J, Gotay CC, Snyder CF. Patient-reported outcomes in cancer: a review of recent research and policy initiatives. CA Cancer J Clin 2007;57:278-300. [Crossref] [PubMed]
- Colt HG, Murgu SD, Korst RJ, et al. Follow-up and surveillance of the patient with lung cancer after curative-intent therapy: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e437S-54S.
- Basch E, Snyder C. Overcoming barriers to integrating patient-reported outcomes in clinical practice and electronic health records. Ann Oncol 2017;28:2332-3. [Crossref] [PubMed]
- Selby JV, Beal AC, Frank L. The Patient-Centered Outcomes Research Institute (PCORI) national priorities for research and initial research agenda. JAMA 2012;307:1583-4. [Crossref] [PubMed]
- Khullar OV, Fernandez FG. Patient-Reported Outcomes in Thoracic Surgery. Thorac Surg Clin 2017;27:279-90. [Crossref] [PubMed]
- Basch E, Abernethy AP, Mullins CD, et al. Recommendations for incorporating patient-reported outcomes into clinical comparative effectiveness research in adult oncology. J Clin Oncol 2012;30:4249-55. [Crossref] [PubMed]
- Gershon RC, Rothrock N, Hanrahan R, et al. The use of PROMIS and assessment center to deliver patient-reported outcome measures in clinical research. J Appl Meas 2010;11:304-14. [PubMed]
- Garcia SF, Cella D, Clauser SB, et al. Standardizing patient-reported outcomes assessment in cancer clinical trials: a patient-reported outcomes measurement information system initiative. J Clin Oncol 2007;25:5106-12. [Crossref] [PubMed]
- Jones RS, Stukenborg GJ. Patient-Reported Outcomes Measurement Information System (PROMIS) Use in Surgical Care: A Scoping Study. J Am Coll Surg 2017;224:245-54.e1. [Crossref] [PubMed]
- Khullar OV, Rajaei MH, Force SD, et al. Pilot Study to Integrate Patient Reported Outcomes After Lung Cancer Operations Into The Society of Thoracic Surgeons Database. Ann Thorac Surg 2017;104:245-53. [Crossref] [PubMed]
- Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg 2015;150:613-9.e2. [Crossref] [PubMed]
- Koller M, Hjermstad MJ, Tomaszewski KA, et al. An international study to revise the EORTC questionnaire for assessing quality of life in lung cancer patients. Ann Oncol 2017;28:2874-81. [Crossref] [PubMed]
- Hollen PJ, Gralla RJ, Kris MG, et al. Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). Eur J Cancer 1993;29A Suppl 1:S51-8. [Crossref] [PubMed]
- Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer 1995;12:199-220. [Crossref] [PubMed]
- Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care 2003;41:1284-92. [Crossref] [PubMed]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-Item Health Survey 1.0. Health Econ 1993;2:217-27. [Crossref] [PubMed]
- Ustun TB, Chatterji S, Kostanjsek N, et al. Developing the World Health Organization Disability Assessment Schedule 2.0. Bull World Health Organ 2010;88:815-23. [Crossref] [PubMed]
- Kleif J, Waage J, Christensen KB, et al. Systematic review of the QoR-15 score, a patient- reported outcome measure measuring quality of recovery after surgery and anaesthesia. Br J Anaesth 2018;120:28-36. [Crossref] [PubMed]
- Straatman J, Joosten PJ, Terwee CB, et al. Systematic review of patient-reported outcome measures in the surgical treatment of patients with esophageal cancer. Dis Esophagus 2016;29:760-72. [Crossref] [PubMed]
- Poghosyan H, Sheldon LK, Leveille SG, et al. Health-related quality of life after surgical treatment in patients with non-small cell lung cancer: a systematic review. Lung Cancer 2013;81:11-26. [Crossref] [PubMed]
- Derogar M, Lagergren P. Health-related quality of life among 5-year survivors of esophageal cancer surgery: a prospective population-based study. J Clin Oncol 2012;30:413-8. [Crossref] [PubMed]
- Courrech Staal EF, Bloemendal KM, Bloemer MC, et al. Oesophageal cancer treatment in a tertiary referral hospital evaluated by indicators for quality of care. Eur J Surg Oncol 2012;38:150-6. [Crossref] [PubMed]
- Bonnetain F, Bouché O, Michel P, et al. A comparative longitudinal quality of life study using the Spitzer quality of life index in a randomized multicenter phase III trial (FFCD 9102): chemoradiation followed by surgery compared with chemoradiation alone in locally advanced squamous resectable thoracic esophageal cancer. Ann Oncol 2006;17:827-34. [Crossref] [PubMed]
- Yun YH, Kim YA, Sim JA, et al. Prognostic value of quality of life score in disease-free survivors of surgically-treated lung cancer. BMC Cancer 2016;16:505. [Crossref] [PubMed]
- Fernando HC, Landreneau RJ, Mandrekar SJ, et al. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg 2015;149:718-25; discussion 725-6. [Crossref] [PubMed]
- Zhao J, Zhao Y, Qiu T, et al. Quality of life and survival after II stage nonsmall cell carcinoma surgery: Video-assisted thoracic surgery versus thoracotomy lobectomy. Indian J Cancer 2015;52 Suppl 2:e130-3. [Crossref] [PubMed]
- Eustache J, Ferri LE, Feldman LS, et al. Enhanced recovery after pulmonary surgery. J Thorac Dis 2018;10:S3755-60. [Crossref] [PubMed]
- Jensen RE, Rothrock NE, DeWitt EM, et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care 2015;53:153-9. [Crossref] [PubMed]
- Refai M, Andolfi M, Gentili P, et al. Enhanced recovery after thoracic surgery: patient information and care-plans. J Thorac Dis 2018;10:S512-6. [Crossref] [PubMed]



