Comparison of specimen adequacy and diagnostic accuracy of a 25-gauge and 22-gauge needle in endobronchial ultrasound-guided transbronchial needle aspiration
Introduction
Endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) is a minimally invasive diagnostic procedure used in the sampling of mediastinal and hilar lymph nodes (1,2). EBUS-TBNA is primarily used in diagnosing and staging lung cancer, but additional applications exist, including evaluation of unexplained mediastinal lymphadenopathy, suspected granulomatous diseases (i.e., sarcoidosis) and lymphoproliferative disorders (2-5). The most recent guidelines from the American College of Chest Physicians (ACCP) and European Society of Thoracic Surgeons (ESTS) recommend EBUS-TBNA as the first-line test for mediastinal staging in non-small cell lung cancer (NSCLC) (1,6).
The literature supports using either a 21-gauge (21G) or 22-gauge (22G) needle in EBUS-TBNA (2). More recently, a 25-gauge (25G) needle has been made available; however, the data to support its use is lacking. The purported advantages of the 25G design include better penetration, greater resistance to deformity and a reduced frequency of specimen contamination with blood (7-10). Studies comparing the 25G and 22G needle in endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) have shown superior diagnostic accuracy of the 25G needle (11).
The aim of this study was to compare specimen adequacy and diagnostic accuracy of the 25G and 22G needle in EBUS-TBNA.
Methods
We retrospectively collected data from patients referred to our institution for EBUS-TBNA between May 2017 and May 2018. This study was approved by the University Hospitals Cleveland Medical Center Institutional Review Board. We included patients who underwent sampling of mediastinal and hilar lymph nodes using either a 25G or 22G needle during EBUS-TBNA. Exclusion criteria were patients age less than 18 years and patients who had more than one needle gauge used during the procedure.
All procedures were performed using Olympus EBUS bronchoscopes and rapid onsite evaluation (ROSE). Either the 25G Boston Scientific (Boston Scientific, USA) or 22G Olympus ViziShot (Olympus America, USA) EBUS-TBNA needle was used. The needle gauge was selected at the discretion of the bronchoscopist. Each lymph node was assessed for adequacy and final diagnosis based on the cytologist report. Specimens were deemed adequate if lymphocytes or atypical cells were present, or a preliminary diagnosis was made. Specimens were considered diagnostic if malignancy or granuloma was identified (12).
Follow-up of non-diagnostic & benign lymphoid tissue specimens
If a specimen was categorized as benign or non-diagnostic, the case was then reviewed for any follow-up diagnostic studies performed. Patients were followed for a minimum of 6 months from the time of initial sampling. According to methods previously described (13,14), applicable follow-up studies include repeat chest computed tomography (CT) or positron emission tomography (PET) scan, surgical sampling (i.e., mediastinoscopy or lymph node dissection), and/or repeat EBUS-TBNA. Patients who received chemotherapy and/or radiation after initial sampling, those who were lost to follow-up, and those who died during the follow-up period were excluded.
If repeat lymph node biopsy did not provide an alternative diagnosis and/or follow-up imaging was stable, the initial specimen was considered a true negative. If the lymph node enlarged on CT, demonstrated increased fluorodeoxyglucose (FDG) avidity on PET, and/or repeat biopsy identified an alternative diagnosis (i.e., malignancy or granuloma), then the initial specimen was considered a false negative.
Statistical analysis
Based on previous studies (7,9), we estimated the difference in diagnostic accuracy between the two groups after matching to be close to 20%. To have 80% power to detect this difference with a statistical significance level of 0.05, we calculated that the number of lymph nodes in each group needed is 80 lymph nodes.
A propensity score matching analysis was performed using “Multivariate and Propensity Score Matching Software with Automated Balance Optimization: The Matching Package for R” with 1:1 ratio, a caliper of 0.2 and the nearest neighbor method (15). Seventy-nine lymph nodes in the 25G group were matched to seventy-nine lymph nodes in the 22G group. The two groups were matched according to age, gender, smoking status, history of COPD, indication for the procedure, lymph node location, lymph node size, number of passes and bronchoscopist. A chi-square test was used to compare sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and diagnostic accuracy between the two groups. All data were reported with the corresponding 95% confidence intervals (CI).
Results
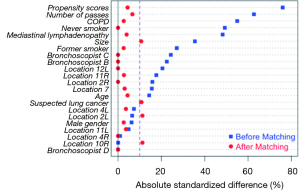
Between May 2017 and May 2018, 112 patients underwent EBUS-TBNA using either a 25G or 22G needle. A total of 242 and 81 lymph nodes were sampled with the 22G and 25G needle, respectively. Baseline characteristics before and after matching are shown in Table 1. Prior to matching, the two groups were significantly different regarding their smoking status, history of COPD, indication for the procedure, lymph node size and number of passes. After matching, the two groups achieved comparable baseline characteristics based on P values and standardized mean differences. A plot of absolute standardized difference is shown in Figure 1.
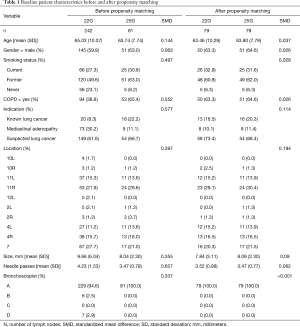
Full table
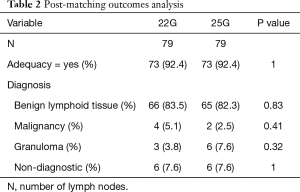
Post-matching outcomes analysis using McNemar’s test is shown in Table 2. An adequate sample was obtained in 73 of 79 lymph nodes (92.4%) in the 25G group and 73 of 79 lymph nodes (92.4%) in the 22G group (P=1). The 25G group diagnosed benign lymphoid tissue in 82.3% (65/79), granuloma in 7.6% (6/79) and malignancy in 2.5% (2/79; two small cell carcinoma). Six lymph nodes in the 25G group were non-diagnostic (7.6%). The 22G group diagnosed benign lymphoid tissue in 83.5% (66/79), granuloma in 3.8% (3/79) and malignancy in 5.1% (4/79; one squamous cell carcinoma and three adenocarcinoma). Six lymph nodes in the 22G group were non-diagnostic (7.6%).
Full table
A total of 71 lymph nodes in the 25G group and 72 lymph nodes in the 22G group were categorized as benign or non-diagnostic. Among them, 46 lymph nodes in the 25G group (64.8%) and 28 lymph nodes in the 22G group (38.9%) were eligible for review of any follow-up diagnostic studies performed. Repeat imaging with CT or PET was obtained in 37 lymph nodes (80.4%) in the 25G group and 13 lymph nodes (46.4%) in the 22G group. Surgery with lymph node biopsy was obtained in 8 lymph nodes (17.4%) in the 25G group and 13 lymph nodes (46.4%) in the 22G group.
Diagnostic performance of the 25G and 22G needle
Of the 46 lymph nodes in the 25G group, a total of 45 nodes met criteria for a true negative. One lymph node grew on repeat CT, and was classified as a false negative. Of the 28 lymph nodes in the 22G group, a total of 26 nodes met criteria for a true negative. Two lymph nodes grew on repeat CT, and were classified as false negatives.
The sensitivity, specificity, PPV, NPV and diagnostic accuracy in the 25G group was 88.9% (95% CI, 51.8–99.7%), 100% (95% CI, 92.1–100%), 100%, 97.8% (95% CI, 87.6–99.7%) and 98.2% (95% CI, 90.1–100%), respectively. The sensitivity, specificity, PPV, NPV and diagnostic accuracy in the 22G group was 77.8% (95% CI, 40–97.2%), 100% (95% CI, 86.8–100%), 100%, 92.9% (95% CI, 79.3–97.8%) and 94.3% (95% CI, 80.8–99.3%), respectively.
There was no statistically significant difference in sensitivity, specificity, PPV, NPV and diagnostic accuracy between the two groups with P values of 1, 1, 1, 0.66 and 0.7, respectively.
Discussion
Through retrospective chart review, our study found no difference in specimen adequacy or diagnostic accuracy of the 25G and 22G needle in EBUS-TBNA. We consider this work to be novel as it addresses a gap in the literature pertaining to the utility of the 25G needle in mediastinal and hilar lymph node sampling. Studies exploring the efficacy of the 25G needle are readily found in the EUS-FNA literature. A meta-analysis by Madhoun et al. found the 25G needle achieved a higher diagnostic accuracy compared to the 22G needle in EUS-FNA of solid pancreatic lesions (11). While EBUS-TBNA and EUS-FNA are targeting different sites, the technology employed is similar. Notably, the two can be combined for sampling of mediastinal lymph nodes in NSCLC to offer a more complete staging procedure (16).
Potential advantages & disadvantages of the 25G needle
The high diagnostic accuracy of EBUS-TBNA is dependent upon successful specimen acquisition and interpretation. The 25G needle is unique in its design, specifically the needle is constructed with a cobalt chromium, whereas most EBUS-TBNA needles (including the 22G) are manufactured with a stainless-steel alloy or nitinol. The difference in needle composition may influence its efficiency, including penetrability, resistance to deformity and durability (10).
Studies comparing different needle sizes in EUS-FNA suggest the advantage of the 25G needle lies in its ability to penetrate firmer lesions (7,8). Although our study excluded patients who had more than one needle used during the procedure, we found success substituting for a smaller needle in instances where the lymph node was difficult to access. This issue of nodal penetrability is often encountered in patients undergoing mediastinal restaging, likely related to fibrosis secondary to prior chemotherapy or radiation (17). The sharpness of the 25G needle also facilitates the to-and-fro movement within the lymph node. This latter point is consequential given that up to 25% of metastases arise in the marginal areas of the node (18).
Another distinct feature of the 25G needle is that fewer specimens are contaminated with blood (9). This is not unusual as prior data have shown larger needles generate bloodier samples (19,20). The presence of blood may obscure diagnostic material, rendering the specimen uninterpretable. This has important implications including failure to ascertain an adequate specimen and potentially increasing the risk of complications through trauma and bleeding (21).
A potential disadvantage of a smaller size needle is the specimen volume is likely to be reduced. Lower quantity specimens are cited as a reason for difficulty in diagnosing lymphoma, where subtyping has important diagnostic and therapeutic implications (4,5). In cases where a diagnosis of lymphoma is suspected or a patient has a history of lymphoma with unexplained mediastinal lymphadenopathy, we tend to favor a larger size needle such as the 21G or 19G.
Additional consideration
After establishing a diagnosis of malignancy, the sample is often sent for additional analysis, including molecular testing (22). EBUS-TBNA can procure ample tissue for such testing; however, operators may be wary of a smaller needle yielding an insufficient sample (23,24). Stoy et al. assessed the success rate of next generation sequencing (NGS) testing from cytology smear specimens using either a 25G or 22G needle. The authors found no significant difference between needles with respect to cytologic assessment of adequacy for small or large panel gene testing. Moreover, the number of needle passes did not differ between the groups (25).
Limitations
There are several limitations to this study. First, the retrospective design has its inherent disadvantages, and while we tried to correct for potential confounders by propensity matching, residual unmeasured confounders may have been missed. Second, most lymph nodes were followed with repeat imaging alone, and did not undergo surgical sampling for histologic confirmation. However, in most cases, patients were followed for more than 6 months making the potential for a missed diagnosis (i.e., malignancy) highly unlikely. Third, the 25G needle was used in a single center by an operator trained in interventional pulmonology, which may limit the generalizability of our results.
Finally, despite a large proportion of our referral base having a known or suspected diagnosis of lung cancer, most lymph nodes were found benign. This discrepancy may be explained by several factors. For instance, the mean lymph node size in both groups was less than 1 cm, and while smaller lymph nodes can harbor metastases, the likelihood of malignancy is significantly lowered (26,27). Alternatively, a patient with suspected lung cancer may undergo a combined procedure with mediastinal staging followed by sampling of the parenchymal lesion. In this case, the mediastinal and hilar nodes are negative, but the lesion may be positive for cancer. Another consideration unique to our geographic region is the high prevalence of fungal infection. Prior studies have shown that in areas of high endemic granulomatous disease, the specificity and NPV of PET/CT is significantly reduced. This is important given that most patients are referred because of suspicious or abnormal findings on initial imaging (28,29).
Conclusions
The 25G needle and 22G needle achieve comparable specimen adequacy and diagnostic accuracy in EBUS-TBNA. Additional factors such as cost, operator experience and suspected diagnosis should be considered prior to needle selection.
Acknowledgments
None.
Footnote
Conflicts of Interest: B Young is a consultant for Pinnacle Biologics, Auris Surgical Robotics and ProVation. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University Hospitals Cleveland Medical Center Institutional Review Board (No. STUDY20180315).
References
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Wahidi MM, Herth F, Yasufuku K, et al. Technical aspects of Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration. Chest 2016;149:816-35. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Kurosu K, et al. The role of EBUS-TBNA for the diagnosis of sarcoidosis--comparisons with other bronchoscopic diagnostic modalities. Respir Med 2009;103:1796-800. [Crossref] [PubMed]
- Korrungruang P, Oki M, Saka H, et al. Endobronchial ultrasound-guided transbronchial needle aspiration is useful as an initial procedure for the diagnosis of lymphoma. Respir Investig 2016;54:29-34. [Crossref] [PubMed]
- Kheir F, Itani A, Assasa O, et al. The utility of endobronchial ultrasound-transbronchial needle aspiration in lymphoma. Endosc Ultrasound 2016;5:43-8. [Crossref] [PubMed]
- De Leyn P, Dooms C, Kuzdzal J, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014;45:787-98. [Crossref] [PubMed]
- Sakamoto H, Kitano M, Komaki T, et al. Prospective comparative study of the EUS guided 25‐gauge FNA needle with the 19‐gauge Trucut needle and 22‐gauge FNA needle in patients with solid pancreatic masses. J Gastroenterol Hepatol 2009;24:384-90. [Crossref] [PubMed]
- Imazu H, Uchiyama Y, Kakutani H, et al. A prospective comparison of EUS-guided FNA using 25-gauge and 22-gauge needles. Gastroenterol Res Pract 2009;2009:546390. [Crossref] [PubMed]
- Yusuf TE, Ho DA, Pavey HM, et al. Retrospective analysis of the utility of endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA) in pancreatic masses, using a 22-gauge or 25-gauge needle system: a multicenter experience. Endoscopy 2009;41:445-8. [Crossref] [PubMed]
- Weston BR, Manoop SB. Optimizing Diagnostic Yield for EUS-Guided Sampling of Solid Pancreatic Lesions: A Technical Review. Gastroenterol Hepatol (N Y) 2013;9:352-63. [PubMed]
- Madhoun MF, Wani SB, Rastogi A, et al. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: A meta-analysis. Endoscopy 2013;45:86-92. [Crossref] [PubMed]
- Yarmus LB, Akulian J, Lechtzin N, et al. Comparison of 21-Gauge and 22-Gauge Aspiration Needle in Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration: Results of the American College of Chest Physicians Quality Improvement Registry, Education, and Evaluation Registry. Chest 2013;143:1036-43. [Crossref] [PubMed]
- Mehta HJ, Tanner NT, Silvestri G, et al. Outcome of Patients with Negative and Unsatisfactory Cytologic Specimens Obtained by Endobronchial Ultrasound Guided Fine Needle Aspiration of Mediastinal Lymph Nodes. Cancer Cytopathol 2015;123:92-7. [Crossref] [PubMed]
- Evison M, Crosbie PA, Morris J, et al. A study of patients with isolated mediastinal and hilar lymphadenopathy undergoing EBUS-TBNA. BMJ Open Respir Res 2014;1:e000040. [Crossref] [PubMed]
- Sekhon J. Journal of Statistical Software. Available online: https://www.jstatsoft.org/v42/i07/. Published 2011. Accessed August 27, 2018.
- Herth FJ, Krasnik M, Kahn N, et al. Combined Endoscopic-Endobronchial Ultrasound-Guided Fine-Needle Aspiration of Mediastinal Lymph Nodes Through a Single Bronchoscope in 150 Patients with Suspected Lung Cancer. Chest 2010;138:790-4. [Crossref] [PubMed]
- Herth FJ, Annema JT, Eberhardt R, et al. Endobronchial Ultrasound with Transbronchial Needle Aspiration for Restaging the Mediastinum in Lung Cancer. J Clin Oncol 2008;26:3346-50. [Crossref] [PubMed]
- Kurimoto N, Osada H, Miyazawa T, et al. Targeting Area in Metastatic Lymph Nodes in Lung Cancer for Endobronchial Ultrasonography-guided Transbronchial Needle Aspiration. J Bronchology Interv Pulmonol 2008;15:134-8.
- Chaddha U, Ronaghi R, Elatre W, et al. Comparison of Sample Adequacy and Diagnostic Yield of 19- and 22-G EBUS-TBNA Needles. J Bronchology Interv Pulmonol 2018;25:264-8. [Crossref] [PubMed]
- Nakajima T, Yasufuku K, Takahashi R, et al. Comparison of 21-gauge and 22-gauge aspiration needle during endobronchial ultrasound-guided transbronchial needle aspiration. Respirology 2011;16:90-4. [Crossref] [PubMed]
- Jeffus SK, Joiner A, Siegel E, et al. Rapid on-site evaluation of EBUS-TBNA specimens of lymph nodes: Comparative analysis and recommendations for standardization. Cancer Cytopathol 2015;123:362-72. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). Non-Small Cell Lung Cancer. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx. Published 2018. Accessed August 27, 2018.
- Folch E, Yamaguchi N, VanderLaan PA, et al. Adequacy of lymph node transbronchial needle aspirates using convex probe endobronchial ultrasound for multiple tumor genotyping techniques in non-small-cell lung cancer. J Thorac Oncol 2013;8:1438-44. [Crossref] [PubMed]
- Navani N, Brown JM, Nankivell M, et al. Suitability of endobronchial ultrasound-guided transbronchial needle aspiration specimens for subtyping and genotyping of non-small cell lung cancer: a multicenter study of 774 patients. Am J Respir Crit Care Med 2012;185:1316-22. [Crossref] [PubMed]
- Stoy SP, Segal JP, Mueller J, et al. Feasibility of Endobronchial Ultrasound-guided Transbronchial Needle Aspiration Cytology Specimens for Next Generation Sequencing in Non–small-cell Lung Cancer. Clin Lung Cancer 2018;19:230-8.e2. [Crossref] [PubMed]
- Garcia-Olivé I, Monso E, Andreo F, et al. Sensitivity of Linear Endobronchial Ultrasonography and Guided Transbronchial Needle Aspiration for The Identification of Nodal Metastasis in Lung Cancer Staging. Ultrasound Med Biol 2009;35:1271-7. [Crossref] [PubMed]
- Wang Memoli JS, El-Bayoumi E, Pastis NJ, et al. Using Endobronchial Ultrasound Features to Predict Lymph Node Metastases in Patients with Lung Cancer. Chest 2011;140:1550-6. [Crossref] [PubMed]
- Sebro R, Aparici CM, Hernandez-Pampaloni M. FDG PET/CT evaluation of pathologically proven pulmonary lesions in an area of high endemic granulomatous disease. Ann Nucl Med 2013;27:400-5. [Crossref] [PubMed]
- Deppen S, Putnam JB, Andrade G, et al. Accuracy of FDG-PET to diagnose lung cancer in a region of endemic granulomatous disease. Ann Thorac Surg 2011;92:428-32. [Crossref] [PubMed]