The upregulated expression of OX40/OX40L and their promotion of T cells proliferation in the murine model of asthma
Introduction
Bronchial asthma (asthma) is a chronic airway inflammatory disease which is concerned with inflammatory cells including T lymphocytes, eosinophils (EOS), and the release of several inflammatory mediators (1,2). T lymphocytes play a central role in asthma. The activation of T cells requires two essential signals, while the OX40/OX40 ligand (OX40L) is a pair of important co-stimulatory signal molecules in the second-signal system (3,4).
The interactions between OX40 and OX40L regulated cytokine production from T cells and promoted antigen-specific T-cell expansion, survival and differentiation. In line with these important modulatory roles, OX40/OX40L interactions had been found to play a vital role in the pathogenesis of multiple inflammatory and autoimmune diseases, making them a research hot spot recently (5,6). In many animal models of diseases, the intervention on OX40/OX40L pathway had achieved certain encouraging effects (7-9). Studies in the murine model of asthma showed that the use of blocking anti-OX40L mAb on intervening OX40/OX40L pathway could decrease airway hyper-responsiveness, lessen the generation of Th2 cytokines, and reduce the production of mucus (10).
OX40 is mainly expressed on activated CD4+ T cells, OX40L is mainly expressed on B lymphocytes, DCs, and macrophage cells (11,12). Siddiqui S et al. (13) found that the expressions of OX40 and OX40L were increased in the lamina propria of asthma patients. The upexpressed OX40/OX40L promoted the polarization of naive T cells, and these polarized T cells produced Th2 cytokines such as IL-4, IL-5, and IL-13, which played an important role in asthma (14). Whether the expression of OX40/OX40L was upregulated in the asthmatic murine model, or the cells upexpressed of OX40/OX40L had some biological functions, thus contributing to the therapeutic effects of blocking anti-OX40L mAb. There are few relevant studies on this area. So our experiment was to provide insights as to the role of OX40/OX40L in the pathogenesis of asthma from the above two aspects.
Materials and methods
Establishment of asthmatic murine model
Specific pathogen free female BALB/c mice, aged 6-8 weeks and weighed 18-22 g, were randomly divided into two groups: control group and asthma group (n=8). The asthma group was sensitized with 200 μL sensitization liquid [20 μg ovalbumin (OVA) in 20 mg of aluminum hydroxide gel] on days 0, 7, 14, and challenged with 5% aerosolized OVA (Sigma, USA) for 45 min in a chamber using a nebulizer (PARI, Germany) on days 24-26. The control group was sensitized and challenged with 0.9% NaCl instead of OVA. The specific methods had slight changes on the basis of Justice JP, Mehta AK et al. (15,16). This study was carried out in strict accordance with the Helsinki Declaration, and was approved by the Medical Ethical Committee of Soochow University.
Measurement of airway hyper-responsiveness
The airway hyper-responsiveness was determined 24 h after the last challenge in the mice. Lung function was recorded as the index of lung resistance (RL) and dynamic compliance (Cdyn), the specific procedures referred to the methods of Pichavant M et al. (17).
Analysis of IL-4 and IFN-γ concentrations in bronchoalveolar lavage fluid (BALF), and OX40, OX40L expressions on BALF cell pellets
The lungs were lavaged three times with 1 mL pre-cooled (4 °C) PBS to collect BALF, which was centrifuged at 2,000 rpm for 5 min to separate liquid from cells. The concentrations of IL-4 and IFN-γ of BALF were measured according to the instruction of the ELISA kits (eBioscience, USA). Total and differential cell counts were determined in the BALF cell pellets. Then, to detect the expressions of OX40 and OX40L, BALF cell pellets were double stained with PE-labeled anti-OX40 mAb (Biolegend, USA)/FITC-labeled anti-CD4 mAb (Immunotech, France), PE-labeled anti-OX40 mAb (Biolegend, USA)/FITC-labeled anti-CD8 mAb (Immunotech, France), PE-labeled anti-OX40L mAb/FITC-labeled anti-CD19 mAb (Immunotech, France) or PE-labeled anti-OX40L mAb/FITC-labeled anti-F4/80 mAb (Biolegend, USA) for 20 min at room temperature. After being washed, the stained cells were analyzed by flow cytometry (Beckman Coulter, USA). PE-anti-mouse IgG (mIgG) (Biolegend, USA) and FITC-anti-mIgG (Immunotech, France) were used as the controls in the experiment.
Collection of blood samples and detection the expressions of OX40, OX40L in peripheral blood mononuclear cell (PBMC)
The blood of the mice was collected in heparin tube. The blood was double stained with PE-labeled anti-OX40 mAb/FITC-labeled anti-CD4 mAb, PE-labeled anti-OX40 mAb/FITC-labeled anti-CD8 mAb, PE-labeled anti-OX40L mAb/FITC-labeled anti-CD19 mAb or PE-labeled anti-OX40L mAb/FITC-labeled anti-F4/80 mAb for 20 min at room temperature. Then the lysing reagent (Beckman Coulter, Brea, CA, USA) was added to the cell suspensions and incubated for another 10 min at 37 °C. After being washed, the stained cells were analyzed by flow cytometry. PE-anti-mIgG and FITC-anti-mIgG were used as the controls.
Characterization of lung histology
Lung sections of 4 μm were cut and stained with hematoxylin-eosin (HE) for the evaluation of pathological changes. In addition, paraffin sections were used to test the protein levels of OX40 (eBioscience, USA), OX40L (R&D, USA) by immunohistochemistry assay. The OX40 and OX40L protein staining intensity was analyzed by Image Pro Plus 6.0 image analysis software at a magnification ×200. And integrated optical density (IOD) was used as relative amount of positive staining described as before (18).
T cell proliferation assay
Spleens were extracted and homogenized by pressing through a 40 μm nylon filter. After lymphocytes from homogenized spleens were isolated by centrifugation over Ficoll, T cells were collected by negatively selecting magnetic beads kit (Miltenyi Biotec, Germany). Then, purified CD3+ T cells (1×105/well) from mice of control group or asthma group were cultured in anti-CD3 mAb (Abcam, USA, 2 μg/mL)-coated 96-well flat-bottom culture plates plus anti-OX40 mAb (Biolegend, USA, 5 μg/mL) or mIgG (Biolegend, USA, 5 μg/mL) as control, the intervention groups were setted as following: control (no anti-CD3 mAb intervention) wells (Con), anti-CD3 mAb intervention wells (CD3), anti-CD3 mAb + IgG (isotype control of anti-OX40 mAb) intervention wells (CD3 + IgG), anti-CD3 mAb + anti-OX40 mAb intervention wells (CD3 + OX40). CCK8 (Dojindo, Japan, 10 μL/well) was added at 68 h and optical density (OD) was measured at 450 nm by a microplate reader (Bio-rad, USA) at 72 h.
Statistical analysis
We analyzed all acquired data by SPSS 17.0 software. The differences among multiple groups were analyzed by one-way ANOVA. The difference between two groups was conducted by independent samples t-test. The difference was judged as statistically significant if P<0.05.
Results
Establishment and evaluation a murine model of asthma
Acute exacerbation of asthma symptoms
During the OVA-sensitized/challenged murine model of asthma was established, all mice in asthma group during nebulization process showed different symptoms of acute exacerbation of asthma: such as piloerection, nodding breathing, rubbing nose, forelimb contraction and raise, etc. While the mice in the control group moved about freely without the above performance.
The increase of airway hyper-responsiveness
The basal values of RL and Cdyn between the control and the asthma groups were similar. When we challenged the mice with saline and 0.025 mg/kg of methacoline, there was no significant difference of RL and Cdyn between the control and asthma group (t=1.86, 1.15; 1.68, 1.28, respectively; P>0.05). When we increased the dose of methacoline and challenged the mice with 0.05, 0.1, 0.2 mg/kg of methacoline, the RL in asthma group was significantly elevated compared with that in control group, while the Cdyn in asthma group was significantly decreased compared with that in control group (t=7.68, 17.10, 18.05; 3.22, 9.97, 13.08, respectively; P<0.05) (Figure 1A,B).
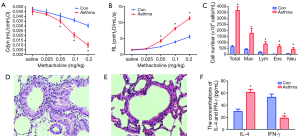
The increase of total and differential cell counts in the BALF cell pellets
After counting and classification of the BALF cell pellets, we found that the total number of cells, EOS, macrophages, lymphocytes, and neutrophils in asthma group were significantly higher than those in control group (t=26.22, 9.65, 9.65, 9.23, 7.35, respectively; P<0.01) (Figure 1C).
Pathological and morphological features of the lung tissues
The histopathology of the lung showed that the organization structure of the peripheral vessels, bronchioles in control group mice were clear. However, in mice of asthma group, there were lots of inflammatory cells around the airways, blood vessels, and lung tissues. The broaden alveolar septa and thickened bronchial wall were also obvious in mice of asthma group (Figure 1D,E).
The concentrations of IL-4 and IFN-γ in BALF of murine model of asthma
In order to validate the murine asthma model we established was successful, we determined the concentrations of IL-4 and IFN-γ in BALF. The result showed that the concentration of IL-4 in BALF of asthmatic mice was significantly higher than that in the control mice (t=14.31, P<0.05); instead, the concentration of IFN-γ in the BALF of asthmatic mice was significantly lower than that in the control group (t=15.28, P<0.01) (Figure 1F).
The upregulated OX40 expression on CD4+ T cells and OX40L expression on B lymphocytes, mononuclear macrophages in PBMC and BALF cell pellets from murine model of asthma
In order to study the expression of OX40 and OX40L on B lymphocytes, mononuclear macrophages, and if there was a difference of OX40 and OX40L expression in different microenvironment. In PBMC, the percentages of CD4+OX40+, CD19+OX40L+ and F4/80+OX40L+ were significantly higher in asthma group than those in control group (t=11.00, 6.71, 5.46; P<0.01), while there was no significant difference between the percentage of CD8+ OX40+ in the two groups (t=0.28, P=0.783) (Table 1). In BALF cell pellets, the percentages of CD4+ OX40+, CD19+ OX40L+ and F4/80+OX40L+ were also obviously higher in asthma compared with those in control group (t=8.44, 4.53, 10.40; P<0.01), while the percentage of CD8+OX40+ showed no significant difference between the two groups (t=0.11, P=0.917) (Table 2).
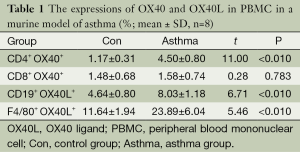
Full table
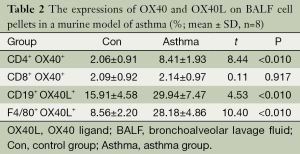
Full table
In asthma group, the percentages of CD4+ OX40+ and CD19+ OX40L+ in BALF cell pellets were higher than those in PBMC (t=5.33, 8.20; P<0.05). The percentages of F4/80+ OX40L+ and CD8+ OX40+ showed no significant difference between the two tissues (t=1.56, 1.30; P>0.05). The results indicated that the cells upregulated OX40/OX40L signal accumulated in the lungs.
OX40/OX40L signal promoted T cell proliferation and secretion of IL-4 and IFN-γ
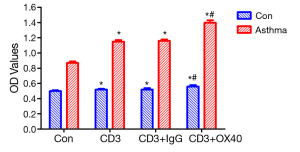
In order to investigate the mechanism of OX40/OX40L signal in T cell proliferation, we conducted T cell proliferation. T cell proliferation assay showed that the OD values of Con, CD3, CD3+IgG, and CD3+OX40 in asthma group were significantly higher than those in control group (t=34.79, 52.14, 48.25, 42.93; P<0.05), indicating that the proliferative capacity of T cells in asthma group was stronger than that in control group. In both control group and asthma group, administration of agnostic anti-OX40 mAb obviously promoted T cell proliferation (P<0.05) (Figure 2).
In addition, we detected the concentrations of IL-4 and IFN-γ in supernatant of T cells. The results showed that the concentrations of IL-4 and IFN-γ which were secreted by T cells in all groups of asthmatic mice were significantly higher than those of the control mice (t=10.55, 20.13, 19.14, 25.46; P<0.01). In both control mice and asthmatic mice, the concentrations of IL-4 and IFN-γ in anti-OX40 mAb intervention group were higher than the other groups (P<0.05) (Figure 3), suggesting that OX40 could help T cells to secrete cytokines.

Upregulated OX40 and OX40L protein expressions in lung tissues
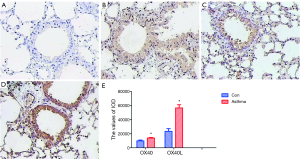
By immunohistochemistry, it was found that OX40 was mainly expressed in the cytoplasm and nucleus of lymphocytes and epithelial cells, while OX40L was mainly expressed in cytoplasm and nucleus of lymphocytes, epithelial cells and macrophages. IOD value of OX40 and OX40L in asthma group were significantly higher than those in control group (t=8.86, 15.46; P<0.01) (Figure 4).
Discussion
The etiology and pathogenesis of asthma are very complex. In view of the limitations of direct human experiments, the animal experiments are very important in the research of etiology, exploration of pathogenesis, and treatment evaluation. The murine asthma model has an irreplaceable role in the study of asthma (19).
Our experiment showed that the symptoms of the mice in asthma group during nebulization process were similar to the symptoms of human acute asthma attack. Compared with the mice of control group, in mice of asthma group the numbers of total cells and EOS of BALF significantly increased and the airway hyper-responsiveness was also obviously upregulated. The concentration of IL-4 in BALF was significantly higher in asthma group than that in control group. All these features showed that the murine asthma model which we established was successful (20).
OX40/OX40L was a pair of very important co-stimulatory molecules in tumor necrosis factor receptor (TNFR) family, and was one of the current research focuses (21). Studies found that in murine asthma model, the use of anti-OX40L mAb to block the OX40/OX40L signaling pathway could relieve the infiltration of EOS, decrease airway hyper-responsiveness, and lessen the generation of Th2 cytokines, which also indicated that OX40/OX40L signal played an important role in the pathogenesis of asthma (10). Whether the above effects of blocking anti-OX40L mAb indicated that there were upregulated of OX40/OX40L signal in the asthmatic murine model, there was little relevant research on this area.
OX40 is mainly expressed on activated CD4+ T cells and also mildly expressed on activation of regulatory T cells, natural killer T cells, and neutrophils (22-27). There is little research on OX40 expression in the murine model of asthma. Our study found that there was low expression of OX40 on CD4+ T cells in PBMC and BALF cell pellets of control group. In the murine asthma model, the expression of OX40 on CD4+ T cells was significantly elevated. There was low expression of OX40 on CD8+ T cells in PBMC and BALF cell pellets of both control and asthma group, and there was no significant difference between the two groups. The phenomenon might also reveal the different status of CD4+ and CD8+ T cells in the pathogenesis of asthma.
OX40L is mainly expressed on B lymphocytes, DCs, and macrophage cells (11,12). In addition, OX40L is also expressed on the surface of Langerhans cells, smooth muscle cells, mast cells, vascular endothelial cells, and CD4+ CD3– secondary cells (27-31). There is also few research on OX40L expression in the murine model of asthma. Our study showed that B lymphocytes and monocyte-macrophage cells upregulated OX40L in the PBMC and BALF cell pellets of asthma mice.
Our study also showed that the percentages of CD4+ OX40+, CD19+ OX40L+ were higher in BALF cell pellets than those in PBMC, which indicated that the cells upregulated OX40/OX40L signal accumulated in the lungs might play an important role in the pathogenesis of asthma.
Except measurement of OX40 and OX40L expressions on some cell populations by flow cytometry in the murine model of asthma, we also analyzed the OX40, OX40L protein expressions in the lung tissues by immunohistochemistry assay. The results showed that compared with the control mice, OX40 and OX40L protein expression levels were increased in the lung tissues of asthmatic mice, which was consistent with the findings of Siddiqui et al. (13). Our results indicated that the membrane forms of OX40 and OX40L were increased in the asthmatic mice. Previously, soluble forms of OX40L were also reported upregulated in asthmatic patients (32,33). These results suggested that there were upregulated OX40/OX40L signaling system in the murine model of asthma.
In order to know whether up-regulation of OX40 on T cells was involved in the pathogenesis of asthma, T cell proliferation assay in vitro was performed. It was found that proliferation and cytokines secretion capacity of T cells in asthmatic mice was stronger than that in control mice. In asthmatic mice, T cells stimulated by OX40 mAb in vitro, have significantly higher capacity of the proliferation and cytokines secretion capacity than other intervention groups, suggesting that upregulated expression of OX40/OX40L signaling pathway could promote T cell proliferation and secretion of cytokines, which contributed to the development of asthma. Whether there were other links, such as the interaction of upregulated membrane form of OX40 and soluble form of OX40L (32,33), it was worth further study.
Conclusions
In conclusion, we successfully established the murine model of asthma, and we found that there were upregulated OX40 expression on CD4+ T lymphocytes, OX40L expression on B lymphocytes, and monocyte-macrophage cells in the asthmatic mice, and increased proteins of OX40, OX40L in lung tissues. We revealed the expressions of OX40 and OX40L more comprehensively in the murine asthmatic model. T cell proliferation assay in vitro showed that OX40/OX40L signaling pathway could promote the proliferation and cytokines secretion capacity of T cells. All these showed that OX40/OX40L signal pathway played an important role in the pathogenesis of asthma.
Acknowledgements
We thanked Hua Liu and Yin-Zi Zhang (Affiliated Hospital of Nantong University, Department of Respiratory Medicine) for measuring the lung function of asthmatic murine model. We thanked Taraka Venkata Pavan for editing this manuscript.
Funding: This work was supported by the social development program of Suzhou City (No: SS0706), youth science and technology project of Suzhou City (No: KJXW2012001), Clinical Key Speciality Project of China, and National Natural Science Foundation of China (No: 81300026, 81100038, 81072451).
Disclosure: The authors declare no conflict of interest.
References
- Adcock IM, Caramori G, Chung KF. New targets for drug development in asthma. Lancet 2008;372:1073-87. [PubMed]
- Asthma Workgroup; Chinese Thoracic Society; Chinese Societ of General Practitioners. Chinese guideline for the prevention and management of bronchial asthma (Primary Health Care Version). J Thorac Dis 2013;5:667-77. [PubMed]
- Beier KC, Kallinich T, Hamelmann E. Master switches of T-cell activation and differentiation. Eur Respir J 2007;29:804-12. [PubMed]
- Cavanagh MM, Hussell T. Is it wise to target the late costimulatory molecule OX40 as a therapeutic target? Arch Immunol Ther Exp (Warsz) 2008;56:291-7. [PubMed]
- Croft M, So T, Duan W, et al. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev 2009;229:173-91. [PubMed]
- Lombardi V, Singh AK, Akbari O. The role of costimulatory molecules in allergic disease and asthma. Int Arch Allergy Immunol 2010;151:179-89. [PubMed]
- Yokouchi H, Yamazaki K, Chamoto K, et al. Anti-OX40 monoclonal antibody therapy in combination with radiotherapy results in therapeutic antitumor immunity to murine lung cancer. Cancer Sci 2008;99:361-7. [PubMed]
- Chen M, Xiao X, Demirci G, et al. OX40 controls islet allograft tolerance in CD154 deficient mice by regulating FOXP3+ Tregs. Transplantation 2008;85:1659-62. [PubMed]
- van Wanrooij EJ, van Puijvelde GH, de Vos P, et al. Interruption of the Tnfrsf4/Tnfsf4 (OX40/OX40L) pathway attenuates atherogenesis in low-density lipoprotein receptor-deficient mice. Arterioscler Thromb Vasc Biol 2007;27:204-10. [PubMed]
- Salek-Ardakani S, Song J, Halteman BS, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med 2003;198:315-24. [PubMed]
- Ito T, Amakawa R, Inaba M, et al. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J Immunol 2004;172:4253-9. [PubMed]
- Karulf M, Kelly A, Weinberg AD, et al. OX40 ligand regulates inflammation and mortality in the innate immune response to sepsis. J Immunol 2010;185:4856-62. [PubMed]
- Siddiqui S, Mistry V, Doe C, et al. Airway wall expression of OX40/OX40L and interleukin-4 in asthma. Chest 2010;137:797-804. [PubMed]
- Linton PJ, Bautista B, Biederman E, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med 2003;197:875-83. [PubMed]
- Justice JP, Borchers MT, Crosby JR, et al. Ablation of eosinophils leads to a reduction of allergen-induced pulmonary pathology. Am J Physiol Lung Cell Mol Physiol 2003;284:L169-78. [PubMed]
- Mehta AK, Gaur SN, Arora N, et al. Effect of choline chloride in allergen-induced mouse model of airway inflammation. Eur Respir J 2007;30:662-71. [PubMed]
- Pichavant M, Goya S, Hamelmann E, et al. Animal models of airway sensitization. Curr Protoc Immunol 2007;Chapter 15:Unit 15.18.
- Liang W, Chen C, Shi J, et al. Disparate effects of eplerenone, amlodipine and telmisartan on podocyte injury in aldosterone-infused rats. Nephrol Dial Transplant 2011;26:789-99. [PubMed]
- Al Heialy S, McGovern TK, Martin JG. Insights into asthmatic airway remodelling through murine models. Respirology 2011;16:589-97. [PubMed]
- Lee MY, Yuk JE, Kwon OK, et al. Anti-inflammatory and anti-asthmatic effects of Viola mandshurica W. Becker (VM) ethanolic (EtOH) extract on airway inflammation in a mouse model of allergic asthma. J Ethnopharmacol 2010;127:159-64. [PubMed]
- Beier KC, Kallinich T, Hamelmann E. Master switches of T-cell activation and differentiation. Eur Respir J 2007;29:804-12. [PubMed]
- Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. EMBO J 1990;9:1063-8. [PubMed]
- So T, Lee SW, Croft M. Immune regulation and control of regulatory T cells by OX40 and 4-1BB. Cytokine Growth Factor Rev 2008;19:253-62. [PubMed]
- Marschner A, Rothenfusser S, Hornung V, et al. CpG ODN enhance antigen-specific NKT cell activation via plasmacytoid dendritic cells. Eur J Immunol 2005;35:2347-57. [PubMed]
- Zaini J, Andarini S, Tahara M, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest 2007;117:3330-8. [PubMed]
- Liu C, Lou Y, Lizée G, et al. Plasmacytoid dendritic cells induce NK cell-dependent, tumor antigen-specific T cell cross-priming and tumor regression in mice. J Clin Invest 2008;118:1165-75. [PubMed]
- Zingoni A, Sornasse T, Cocks BG, et al. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol 2004;173:3716-24. [PubMed]
- Burgess JK, Carlin S, Pack RA, et al. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol 2004;113:683-9. [PubMed]
- Nakae S, Suto H, Iikura M, et al. Mast cells enhance T cell activation: importance of mast cell costimulatory molecules and secreted TNF. J Immunol 2006;176:2238-48. [PubMed]
- Kashiwakura J, Yokoi H, Saito H, et al. T cell proliferation by direct cross-talk between OX40 ligand on human mast cells and OX40 on human T cells: comparison of gene expression profiles between human tonsillar and lung-cultured mast cells. J Immunol 2004;173:5247-57. [PubMed]
- Gaspal FM, Kim MY, McConnell FM, et al. Mice deficient in OX40 and CD30 signals lack memory antibody responses because of deficient CD4 T cell memory. J Immunol 2005;174:3891-6. [PubMed]
- Ezzat MH, Imam SS, Shaheen KY, et al. Serum OX40 ligand levels in asthmatic children: a potential biomarker of severity and persistence. Allergy Asthma Proc 2011;32:313-8. [PubMed]
- Lei W, Zhu CH. SOX40L: an important inflammatory mediator in adult bronchial asthma. Ann Acad Med Singapore 2012;41:200-4. [PubMed]


