
High-volume hydration for the prevention of acute kidney injury after cardiac surgery
Acute kidney injury (AKI) is a serious complication after cardiac surgery, occurring between 5% to 30% of patients, according to the used definition (1-16). In 6% to 20% of patients experiencing AKI, dialysis is required (11,12). Development of AKI leads to an increase in mortality, both at short- and long-term follow-up (1-18). In particular, it has been showed that 12% to 64% of patients experiencing AKI die, as compared to 1% to 5% of patients not developing AKI (11-15). This mortality risk increases also with minor serum creatinine elevations, exceeding 50% in cases requiring hemodialysis (15). Moreover, AKI has a critical impact on non-renal morbidity, as it is associated after cardiac surgery with a higher rate of respiratory insufficiency, infections, sepsis, and gastrointestinal bleeding (1-12).
Several risk factors for post-operative AKI have been recognized, including age, pre-existing chronic kidney disease (CKD), heart failure history, and/or reduced left ventricular ejection fraction (LVEF), diabetes, protracted cardiopulmonary bypass (CPB) time, and recent administration of nephrotoxic agents (1-16). Notably, in patients with CKD, AKI may occur in about 50% of patients after cardiac surgery and it is associated with an almost ten times increased perioperative mortality (16).
Factors contributing to AKI after cardiac surgery include hemodynamic and metabolic alterations, inflammation, exogenous toxins, vasoconstrictors release, and the interactions between blood components and artificial membranes (18). They all contribute to renal vasoconstriction and ischemia. Thus, the unique characteristics of cardiac surgery using CPB markedly increase AKI risk when compared with other clinical and surgical settings (19-21), mainly due to aorta cross-clamping and exogenous blood product transfusion and vasopressors.
As patients at high-AKI risk can be easily identified before cardiac surgery, the best AKI treatment is its prevention, since its management is only supportive in nature, with renal replacement therapy being the mainstay of treatment for severe renal failure. Among prophylactic measures, hydration represents the cornerstone. Indeed, the kidney receives 20% to 25% of total cardiac output and its medullary portion of the nephrons is particularly vulnerable to ischemia. This area is maintained at low oxygen tension, whereas its active sodium transport is associated with high metabolic activity and oxygen requirements. Thus, not adequate circulating volume leads to renal hypoperfusion and elicits neurohumoral responses, promoting further kidney vasoconstriction. On the other hand, vigorous hydration may counteract these detrimental phenomena by optimizing systemic hemodynamics, increasing renal flow, and preserving medullary perfusion (22). Of note, a previous meta-analysis of 4,220 surgical patients showed that perioperative hemodynamic optimization, obtained by fluids and inotropes reduce the incidence of postoperative AKI (21).
The results of the study published in this issue of Journal of Thoracic Disease by Lim et al. (23) provided a further contribution to the evolving literature on the potential protective role of hydration against AKI in cardiac surgery. The authors addressed whether high-volume saline administration is associated with a lower AKI incidence in their retrospective registry of 1,740 patients undergoing cardiac surgery with CPB. They found that high-volume saline administration (arbitrarily defined as more than 1 liter infused in the first 48 postoperative hours) was not associated to a significant different AKI risk (9% vs. 9%), need for renal replacement therapy (6% vs. 5%), or in-hospital mortality (4% vs. 4%), when compared to patients receiving low-volume saline (<1 liter/48 hours; mean 0.5 liters). Strengths of this analysis are an appropriate sample size registry with well-characterized data collection. Moreover, the authors aimed at reducing confounding factors in the assessment of the effect of saline after cardiac surgery with propensity score-matched analysis, which confirmed the study results, even after adjustment for major covariates. However, some limitations warrant mention. This was a single-center study, limiting the generalizability of the results due to potentially unique practice patterns at the study site. Moreover, given its retrospective nature, the results of the study should be considered hypothesis generating only.
The neutral conclusions of this study should be examined according to some relevant clinical aspects. First, in this report, as well as in many previous studies, intravenous saline infusion was started after intensive care unit arrival and not before cardiac surgery. When renal function acutely decreases, serum creatinine rises slowly, usually within days and, although AKI typically occurs during CPB, it is usually recognized later. For this reason, creatinine concentration is a delayed measure of kidney dysfunction in the acute setting (24). Therefore, post-operative hydration does not represent a “truly preventive” measure, but it should be considered only a “renal supportive” strategy which, at best, reduces the severity of the ongoing AKI and facilitates kidney function recovery. Second, the mean saline volume administered in the high-volume group was 2 liters over 48 hours, corresponding to an infusion rate of about 40 mL/hour. If we consider that daily water requirement is 2.5–3 liters/day, corresponding to about 100–120 mL/hour, the fluid amount considered in this study cannot be defined as a high-volume hydration. Rather, it represents a “prudential” (safe more than effective) hydration regimen. Thus, whether a preventive and vigorous saline hydration is beneficial against AKI after cardiac surgery remains unclear, thus far. Lastly, another point that needs to be addressed is the inclusion in this registry of patients at low AKI risk. Of note, AKI incidence in this study was 9–11% (by RIFLE and KDIGO criteria, respectively), a figure lower than that observed in most cardiac surgery series (1-16,25) (Table 1). This can be explained by the fact that the mean age of the study population was 59 years, mean LVEF and an estimated glomerular filtration rate were normal and the mean aortic cross-clamp time was less than 100 minutes. As advanced age and reduced cardiac and renal function are the most critical risk factors for AKI, patients presenting with these characteristics should be identified before cardiac surgery, and they represent the ideal subset of patients in whom to implement prophylactic measures. Accordingly, in the meta-analysis by Brienza et al. (21), postoperative AKI rate was significantly reduced only in studies in which fluids were administered in high-risk patients.
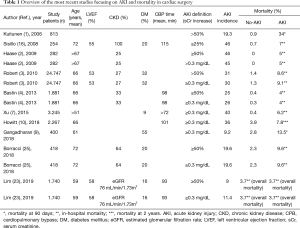
Full table
In conclusion, given its detrimental clinical relevance, every effort should be made to prevent AKI after cardiac surgery. Future trials should deal with AKI as main outcome, share the same definition, and should be performed in patients at high AKI risk. In these patients, a preventive strategy based on an early, preoperative, high-volume fluid administration should be evaluated, in terms of AKI incidence and severity, and of potential morbidity and mortality improvement. Taken together all these considerations, the benefits of a prophylactic high-volume hydration in cardiac surgery still warrant investigation.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Kuitunen A, Vento A, Suojaranta-Ylinen R, et al. Acute renal failure after cardiac surgery: Evaluation of the RIFLE classification. Ann Thorac Surg 2006;81:542-6. [Crossref] [PubMed]
- Haase M, Bellomo R, Matalanis G, et al. A comparison of the RIFLE and Acute Kidney Injury Network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort study. J Thorac Cardiovasc Surg 2009;138:1370-76. [Crossref] [PubMed]
- Robert AM, Kramer RS, Dacey LJ, et al. Cardiac surgery-associated acute kidney injury: A comparison of two consensus criteria. Ann Thorac Surg 2010;90:1939-43. [Crossref] [PubMed]
- Bastin AJ, Ostermann M, Slack AJ, et al. Acute kidney injury after cardiac surgery according to Risk/Injury/Failure/Loss/End-stage, Acute Kidney Injury Network, and Kidney Disease: Improving Global Outcomes classifications. J Crit Care 2013;28:389-96. [Crossref] [PubMed]
- Dardashti A, Ederoth P, Algotsson L, et al. Incidence, dynamics, and prognostic value of acute kidney injury for death after cardiac surgery. J Thorac Cardiovasc Surg 2014;147:800-7. [Crossref] [PubMed]
- Li Z, Cai L, Liang X, et al. Identification and predicting short-term prognosis of early cardiorenal syndrome type 1: KDIGO is superior to RIFLE or AKIN. PLoS One 2014;9:e114369. [Crossref] [PubMed]
- Xu JR, Zhu JM, Jiang J, et al. Risk Factors for Long-Term Mortality and Progressive Chronic Kidney Disease Associated With Acute Kidney Injury After Cardiac Surgery. Medicine (Baltimore) 2015;94:e2025. [Crossref] [PubMed]
- Seelhammer TG, Maile MD, Heung M, et al. Kinetic estimated glomerular filtration rate and acute kidney injury in cardiac surgery patients. J Crit Care 2016;31:249-54. [Crossref] [PubMed]
- Gangadharan S, Sundaram KR, Vasudevan S, et al. Predictors of acute kidney injury in patients undergoing adult cardiac surgery. Ann Card Anaesth 2018;21:448-54. [Crossref] [PubMed]
- Howitt SH, Grant SW, Caiado C, et al. The KDIGO acute kidney injury guidelines for cardiac surgery patients in critical care: a validation study. BMC Nephrol 2018;19:149. [Crossref] [PubMed]
- Thiele RH, Isbell JM, Rosner MH. AKI associated with cardiac surgery. Clin J Am Soc Nephrol 2015;10:500-14. [Crossref] [PubMed]
- Wang Y, Bellomo R. Cardiac surgery-associated acute kidney injury: risk factors, pathophysiology and treatment. Nat Rev Nephrol 2017;13:697-711. [Crossref] [PubMed]
- Hix JK, Thakar CV, Katz EM, et al. Effect of off-pump coronary artery bypass graft surgery on postoperative acute kidney injury and mortality. Crit Care Med 2006;34:2979-83. [Crossref] [PubMed]
- Mangano CM, Diamondstone LS, Ramsay JG, et al. Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resources utilization. Ann Intern Med 1998;128:194-203.78.
- Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004;15:1597-605. [Crossref] [PubMed]
- Sisillo E, Ceriani R, Bortone F, et al. N-acetylcysteine for prevention of acute renal failure in patients with chronic renal insufficiency undergoing cardiac surgery. A prospective, randomized, clinical trial. Crit Care Med 2008;36:81-6. [Crossref] [PubMed]
- Hillis GS, Croal BL, Buchan KG, et al. Renal function and outcome from coronary artery bypass grafting. Impact on mortality after a 2.3-year follow-up. Circulation 2006;113:1056-62. [Crossref] [PubMed]
- Parolari A, Pesce LL, Pacini D, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg 2012;93:584-91. [Crossref] [PubMed]
- Marenzi G, Cabiati A, Bertoli SV, et al. Incidence and relevance of acute kidney injury in patients hospitalized with acute coronary syndromes. Am J Cardiol 2013;111:816-22. [Crossref] [PubMed]
- Cardinale D, Cosentino N, Moltrasio M, et al. Acute kidney injury after lung cancer surgery: Incidence and clinical relevance, predictors, and role of N-terminal pro B-type natriuretic peptide. Lung Cancer. 2018;123:155-9. [Crossref] [PubMed]
- Brienza N, Giglio MT, Marucci M, et al. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta-analytic study. Crit Care Med 2009;37:2079-90. [Crossref] [PubMed]
- Briguori C, Condorelli G. Hydration in contrast-induced acute kidney injury. Lancet 2014;383:1786-8. [Crossref] [PubMed]
- Lim JY, Kang PJ, Jung HS. Effect of high- versus low-volume saline administration on acute kidney injury after cardiac surgery. J Thorac Dis 2018;10:6753-62. [Crossref] [PubMed]
- Marenzi G, Cosentino N, Bartorelli AL. Acute kidney injury in patients with acute coronary syndromes. Heart 2015;101:1778-85. [Crossref] [PubMed]
- Borracci RA, Macias Miranda J, Ingino CA. Transient acute kidney injury after cardiac surgery does not independently affect postoperative outcomes. J Card Surg 2018;33:727-33. [Crossref] [PubMed]


