
Platelet-leukocyte rich gel application in the prevention of deep sternal wound problems after cardiac surgery in obese diabetic patients
Introduction
Although infrequent, deep sternal wound problems (DSWP) such as mediastinitis or a non-fusing sternum following sternotomy for cardiac surgery have a significant impact on patient recovery. Extended hospital stay and repeated surgery is often required, increasing the risk for additional complications. Thus, DSWP are associated with an increased morbidity and mortality (1,2). It has been shown that certain groups of cardiac surgery patients, such as obese or diabetic patients, have an increased risk for DSWP (3,4).
The risk for DSWP can conceivably be reduced by preventing infection or boosting tissue healing in the sternal wound. Both might be accomplished by the local application of concentrated activated platelets, as in recent years it has become apparent that platelets play a pivotal role in wound healing processes beyond the well-known closing of the endothelial defect. Numerous growth factors and chemoattractants are excreted from activated platelets, in sufficient amounts to stimulate vascular proliferation and attract tissue stem cells (5). In addition platelets also appear to have anti-microbial properties (6).
Centrifugal-based platelet concentration methods have been developed separating whole blood in a red blood cell (RBC) layer, a platelet rich plasma (PRP) layer and a platelet poor plasma (PPP) layer. The PRP layer also contains a high concentration of leukocytes, which might provide additional protection against infection (7). Activation of the concentrated platelets brings about the excretion of growth factors, and induces the platelet suspension to jellify. This PRP gel (PLG) can be applied between the sternal halves, immediately prior to closing the sternum, to enhance the healing processes.
Methods
Patients
PLG was applied to all patients receiving elective cardiac surgery involving a median sternotomy and cardiopulmonary bypass, with a body mass index (BMI) >30 and diagnosed diabetes mellitus type 2 (DM 2). The reference group constituted of all elective patients with a BMI >30 and DM 2 receiving on-pump cardiac surgery involving a median sternotomy operated upon in the 2-year period before the application of PRP. Exclusion criteria for both groups were additional treatments possibly affecting DSWP incidence, most notably resternotomy and additional antibiotic treatment.
This study has been carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent (Research Ethics Committee of the Radboud University Nijmegen Medical Centre, CMO-registration number 2018-4304).
PRP preparation and application
Blood (100 mL) was aspirated from the central venous line prior to heparin administration and before start of surgery, to minimize the activation of the collected platelets. It is important not to activate the platelets prior to application, as the excretion of growth factors following activation peaks within 10 minutes, and is exhausted in 100 minutes (8). Blood was anticoagulated with ACD-A (Fresenius Kabi NL, The Netherlands) using a ratio of 9 vol. blood to 1 vol. ACD-A. The collected blood was centrifuged (Angel whole blood separation system, Arthrex, Son en Breugel, The Netherlands) and separated in an RBC component, a PRP component, and a PPP component. The spin protocol used for a volume of 100 mL whole blood: 3,667 rpm 7 min 41 s; 3,000 rpm 4 min 40 s, yielding PRP with a hematocrit of 7%, as recommended by the manufacturer.
PRP was diluted with PPP to a final volume of 8–10 mL, and stored at room temperature. For PLG patients 1 to 93, 12 mL of the PPP was used to produce thrombin using the Activat device (Arthrex, The Netherlands) according to the manufacturer’s protocol, and kept at room temperature. For PLG patients 94 to 144, due to production discontinuation, the Activat disposable was unavailable. As alternative the Clotalyst device (Zimmer Biomet, Dordrecht, The Netherlands) was used, which required an additional 11 mL whole blood, anticoagulated with 1 mL ACD-A, to produce autologous thrombin according to manufacturer’s protocol. Clotalyst trombin was kept at 4 °C until use. Remaining PPP and RBC were returned to the patient during cardiopulmonary bypass.
Immedialtely prior to sternal closure, thrombin was added to the PRP in a ratio of 1:10. As soon as the activated PRP started to jellify (needing 20–60 s), it was administered using a blunt large bore needle (Monoject 15 G × 1−1/2" blunt cannula, Covidien/Medtronic, Zaltbommel, The Netherlands) between the sternal halves which were kept approximately 1 mm apart to allow the gel to get stuck between, after which the sternal halves were joined. Care was taken not to remove any of the activated PRP by cell saver suction during closure of the sternotomy wound.
Cardiac surgery
Surgery was performed according to local guidelines. Patients were heparinized using an initial dose of 300 IU/kg unfractionated heparin (LEO pharma, Breda, The Netherlands). During surgery the activated clotting time (ACT) was maintained at least 480 s. The antibiotic prophylaxis changed during the second year of PLG treatment. The reference group and PLG patients 1–60 were treated with cefuroxim 1,500 mg at least 30 min. before incision, which was repeated after 4 hours of surgery or blood loss >2 Liters. PLG patients 60–144 were treated with cefazoline (Eurocept, Ankeveen, The Netherlands) 2,000 mg administered 15−60 min. before incision, which was repeated after 4 hours of surgery or more than 500 mL administered cell saver blood. Following sternotomy, the use of bonewax on the sternum was avoided, as this might form an intrasternal barrier and interfere with PRP augmented healing. Filtered CO2 flush (2 L/min) in the thoracic cavity was administered during valve or aortic surgery to prevent the entrapment of intracardial air emboli, starting approximately 10–15 minutes prior to closing the heart, and stopping after aortic clamp removal. Targeted flow rates during cardiopulmonary bypass were 2.6 L/min/m2, yielding a venous saturation of 70−80%. The sternum was closed using 6 to 8 single steel wires or single steel wires combined with Zipfix, an implantable cable tie system (DePuy Synthes, The Netherlands), at the surgeon’s discretion. After surgery protamin hydrochloride was administered (in a 1:1 ratio with administered heparin) to neutralize residual heparin activity.
Statistics
To compare the perioperative parameters, we used for continuous measurements the 2-tailed two-sample t test and for binomial values the N-1 two-proportion test. To compare DSWP incidence between the reference group and the PLG group, the chi square test was applied.
Results
For a four-year period (2014–2017) PLG was applied to all (n=166) patients (BMI >30, DM 2) receiving elective cardiac surgery involving a median sternotomy and cardiopulmonary bypass. Twenty-two patients received a resternotomy or an additional surgical intervention which could affect the incidence of DSWP, such as the application of gentamycin pads, and were excluded. Thus, the PLG group consisted of 144 patients. The reference group constituted of all (n=129) elective patients (BMI >30, DM 2) receiving on-pump cardiac surgery involving a median sternotomy operated upon in the 2-year period (2012–2013) before the application of PRP. We excluded nine patients from this group for receiving a resternotomy and two patients who were lost to follow up. Thus, the reference group consisted of 118 patients.
To determine whether the reference group and the PLG group were equally vulnerable to develop DSWP, we compared both groups for several pre- and perioperative parameters which might affect the incidence of DSWP (3,4,9,10) (Table 1).
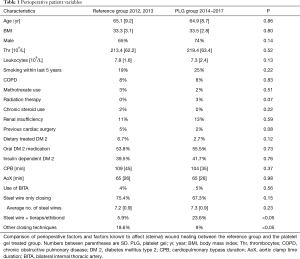
Full table
DSWP were considered present when sternal instability or dehiscence was reported in the 6-week follow-up report or later, or when thorax wound infections requiring surgical interventions excluding superficial ones were reported. In the reference group 13 out of 118 patients developed DSWP, in the PLG treated group 6 out of 144 patients developed DSWP (Table 2). In the reference group DSWP occurred in 11% of the obese, diabetic patients. Following the application of PLG this risk was reduced to 4.2%.

Full table
The PLG group consisted of 144 patients. Twenty-two patients were excluded postoperatively from the PLG group for various reasons, but were treated with PLG nonetheless. PLG was thus applied to 166 patients for a total cost of €58,100. Of the 144 patients in the PLG group 6 (4,2%) developed DSWP. Without PLG treatment and a risk of 11%, there would have been approximately 15–16 patients with DSWP. This means that the cost of each avoided DSWP in this study is €3,875−€3,630.
Discussion
A varying incidence of DSWP following cardiac surgery has been reported, ranging from 0.4–3% in unselected patients (3,10). In our institution in the years 2011 to 2016 the incidence of DSWP in all cardiac surgery patients was 1.3%, which corresponds to other reports. Several techniques have been shown to reduce the incidence of DSWP, such as the application of prophylactic vancomycin (11), or xyphoid-sparing sternotomy (12). We have focused on the effect of sternal PRP application on DSWP incidence.
Prior to the application of PLG, the characteristics of patients who had developed DSWP were examined, to establish an optimal target patient population. Hypertension, obesity (BMI >30) and DM 2, risk factors for the development of DSWP (3,4,9), were determined. The patient group with both obesity and DM 2 included 42% of the total number of patients with DSWP, while this group was only 4.3% of the total patient population. After evaluation it was decided to apply PLG to this patient group, to ensure feasibility and maximize the potential cost benefit ratio.
With the introduction of an additional treatment detrimental side effects can occur, for the sternal application of PLG most notably an increase in infections. Therefore, postoperative patient temperatures, leukocyte counts and C-reactive protein levels were monitored during the first year and 9 months of PLG application (n=67), and compared to the reference group. No differences were observed and it was concluded no significant additional risk for infection was introduced with this technique. In addition, we did not want to use substances potentially inducing (auto) immune reactions, such as bovine thrombin. Therefore, autologous thrombin was used to achieve platelet activation.
The main limitation of this report is the use of a retrospective reference group. This was accepted after careful deliberation, firstly since we felt PLG to be beneficial and did not want to deny it to high risk patients, secondly since the low incidence of DSWP would require a large patient population to set up a prospective controlled study with sufficient power.
To determine possible confounding factors between the reference patient group and the PLG patient group, we have taken into account the sternal closing techniques, the use of BITA grafting and the applied antibiotic prophylaxis. There was no difference in BITA use between the reference group and the PRP group. Antibiotic regimen was changed during this study, following a change in national guidelines, from the first generation β-lactam antibiotic ceforuxim to the second generation β-lactam antibiotic cefazoline, albeit with similar perioperative protocols. The reference group and patients 1–60 of the PLG group received ceforuxim, patients 61–144 of the PLG group received cefazoline. We found no differences in DSWP incidence following the change in antibiotic prophylaxis. Another factor which might have an effect on the prevalence of DSWP is the sternal closure technique. In the reference group sternal closure was mostly performed by using single steel wires only compared to the PLG group. In the PLG group there was increased usage of single steel wires combined with Zipfix or ethibond sutures. The rationale being that substituting distal steel wiring for Zipfix or ethibond improves sternal stability and reduces local pressure by thin steel wires. Between both groups there was no significant difference in the number of steel wires used per sternum when the closing technique was steel wire only. There is no apparent correlation between DSWP and the applied closing technique in this study, although others found a reduction of DSWP when using Zipfix combined with steel wiring compared to steel wiring only (11). Of the 13 DSWP cases in the reference group, 11 sternums were closed using steel wiring only (84%), 2 sternums were closed using steel combined with Zipfix or ethibond (16%). Of the 6 patients with DSWP in the PLG group 3 sternums were closed with steel wiring only (50%), 2 sternums with steel and Zipfix or ethibond (33%), and 1 sternum was closed using Zipfix only (16%). Tentative P value calculations show no significant differences in applied closing techniques between the reference group and patients with DSWP from that group (P=0.45) and between the PLG treated group and the patients with DSWP from that group (P=0.40). However, the low number of DSWP likely prevents a possible association to reach significance.
Other studies have been performed to evaluate the effect of activated PRP on sternal wound infections in unselected cardiac surgery patients. Although also with the limitation of a retrospective observational design, several studies suggest beneficial effects of PLG application on deep and superficial sternal wound infections (12-14). In contrast, several prospective studies found no effect of PLG application in high risk (15) or consecutive cardiac patients (16), but as in these studies the control group and the study group were smaller compared to our patient groups, a significant effect might have been more difficult to detect. Another prospective study, with a population size comparable to our study, used patient groups with one or more risk factors for DSWP (17). In contrast, our patient groups exhibited two risk factors, both DM 2 and BMI >30, resulting in a patient population with on average a higher, and more homogenous risk profile for DSWP, thus a decrease in DSWP might have been easier to detect. In addition, PRP can be produced and activated via various protocols from various manufacturers. Differences in PRP preparation methods, potentially concentrating different platelet populations, could complicate the comparison of results between studies, as it has been reported that different platelet types exhibit different activities (18).
A randomized controlled trial would provide proof of the effectiveness of PLG in the prevention of DSWP in obese DM 2 cardiac patients. There appears, however, at present no justifiable reason to approve of such a study in our clinic, as the added risk of the PLG treatment appears negligible, the overall cost of PLG treatment is far less than the cost of even a few DSWP treatments and the results suggest a significant decrease in DSWP incidence. The high risk of complications and comorbidities of DSWP treatment, with extended hospital stay and additional surgical procedures, warrants the preventive use of PLG in patients at high risk of a disturbed sternal wound healing.
Acknowledgements
The authors thank all involved for the collecting of samples, and assisting in the application of the PLG.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study has been carried out in the Netherlands in accordance with the applicable rules concerning the review of research ethics committees and informed consent (Research Ethics Committee of the Radboud University Nijmegen Medical Centre, CMO-registration number 2018-4304).
References
- Lu JC, Grayson AD, Jha P, et al. Risk factors for sternal wound infection and mid-term survival following coronary artery bypass surgery. Eur J Cardiothorac Surg 2003;23:943-9. [Crossref] [PubMed]
- Borger MA, Rao V, Weisel RD, et al. Deep sternal wound infection: risk factors and outcomes. Ann Thorac Surg 1998;65:1050-6. [Crossref] [PubMed]
- Balachandran S, Lee A, Denehy L, et al. Risk Factors for Sternal Complications After Cardiac Operations: A Systematic Review. Ann Thorac Surg 2016;102:2109-17. [Crossref] [PubMed]
- Diez C, Koch D, Kuss O, et al. Risk factors for mediastinitis after cardiac surgery - a retrospective analysis of 1700 patients. J Cardiothorac Surg 2007;2:23. [Crossref] [PubMed]
- Blair P, Flaumenhaft R. Platelet alpha-granules: basic biology and clinical correlates. Blood Rev 2009;23:177-89. [Crossref] [PubMed]
- Tohidnezhad M, Varoga D, Podschun R, et al. Thrombocytes are effectors of the innate immune system releasing human beta defensin-3. Injury 2011;42:682-6. [Crossref] [PubMed]
- Bielecki TM, Gazdzik TS, Arendt J, et al. Antibacterial effect of autologous platelet gel enriched with growth factors and other active substances: an in vitro study. J Bone Joint Surg Br 2007;89:417-20. [Crossref] [PubMed]
- Arnoczky SP, Sheibani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc Rev 2013;21:180-5. [Crossref] [PubMed]
- Milano CA, Kesler K, Archibald N, et al. Mediastinitis after coronary artery bypass graft surgery. Risk factors and long-term survival. Circulation 1995;92:2245-51. [Crossref] [PubMed]
- Mauermann WJ, Sampathkumar P, Thompson RL. Sternal wound infections. Best Pract Res Clin Anaesthesiol 2008;22:423-36. [Crossref] [PubMed]
- Stelly MM, Rodning CB, Stelly TC. Reduction in deep sternal wound infection with use of a peristernal cable-tie closure system: a retrospective case series. J Cardiothorac Surg 2015;10:166. [Crossref] [PubMed]
- Patel AN, Selzman CH, Kumpati GS, et al. Evaluation of autologous platelet rich plasma for cardiac surgery: outcome analysis of 2000 patients. J Cardiothorac Surg 2016;11:62. [Crossref] [PubMed]
- Serraino GF, Dominijanni A, Jiritano F, et al. Platelet-rich plasma inside the sternotomy wound reduces the incidence of sternal wound infections. Int Wound J 2015;12:260-4. [Crossref] [PubMed]
- Trowbridge CC, Stammers AH, Woods E, et al. Use of platelet gel and its effects on infection in cardiac surgery. J Extra Corpor Technol 2005;37:381-6. [PubMed]
- Litmathe J, Philipp C, Kurt M, et al. The use of autologous platelet gel (APG) for high-risk patients in cardiac surgery -- is it beneficial? Perfusion 2009;24:381-7. [Crossref] [PubMed]
- Vang SN, Brady CP, Christensen KA, et al. Autologous platelet gel in coronary artery bypass grafting: effects on surgical wound healing. J Extra Corpor Technol 2007;39:31-8. [PubMed]
- Dörge H, Sellin C, Bury MC, et al. Incidence of deep sternal wound infection is not reduced with autologous platelet rich plasma in high-risk cardiac surgery patients. Thorac Cardiovasc Surg 2013;61:180-4. [Crossref] [PubMed]
- Milants C, Bruyère O, Kaux JF. Responders to Platelet-Rich Plasma in Osteoarthritis: A Technical Analysis. Biomed Res Int 2017;2017:7538604. [Crossref] [PubMed]


