
Efficacy and safety profile of roflumilast in a real-world experience
Introduction
Chronic obstructive pulmonary disease (COPD) is currently the fourth leading cause of death in the world, but is projected to be the 3rd leading cause of death by 2020 (1). Long-acting inhaled bronchodilators with or without inhaled corticosteroids are central to the management of patients with moderate to severe COPD.
Roflumilast is an orally administered phosphodiesterase enzyme 4 (PDE4) inhibitor indicated as a treatment to reduce the risk of COPD exacerbations in patients with severe COPD associated with chronic bronchitis and a history of frequent exacerbations. Its principal action is to reduce inflammation by inhibiting the breakdown of intracellular cyclic AMP. The beneficial effects of roflumilast have been reported to be greater in COPD patients with a prior history of hospitalizations due to acute exacerbations (2,3).
Several clinical studies have demonstrated that roflumilast improves lung function and reduces the risk of exacerbation, and inhibition of airway inflammation might contribute to the beneficial effect of roflumilast on COPD. On the other hand, it is still difficult to judge whether or not the benefits of roflumilast outweigh the side effects. PDE4 inhibitors lead to more adverse events (AEs) than inhaled medications in COPD (3). The most frequent AEs are diarrhea, reduced appetite, weight loss, abdominal pain, sleep disturbance and headache. A recent meta-analysis showed that roflumilast significantly increased AEs such as diarrhea (RR 2.945, P<0.001) and weight loss (RR 3.814, P<0.001) (4). Randomized controlled clinical trials (RCTs) do not always reflect a real-world population. In real-world clinical experience, reported side effect and drug discontinuation rates seem to be higher than the figures reported in RCTs (5-8), but real-world studies about the use of roflumilast are still limited.
Our aim was to assess the safety of roflumilast in the treatment of stable patients with severe COPD in a real-world setting. We also analyzed the effect of roflumilast on exacerbation and hospitalization rates in COPD.
Methods
From August 2014 to August 2017, medical records of all COPD patients who were prescribed roflumilast therapy in Akdeniz University Hospital were analyzed retrospectively. Only the outpatients whose treatment for COPD, other than addition of roflumilast (500 µg tablet, once daily), was not changed were included in the study. The patients were on stable state when the roflumilast therapy was prescribed. The present study was conducted according to the principles stated in the Declaration of Helsinki, and the local ethics committee approved the study protocol (date: July 25, 2018 and issue number: 528). A COPD diagnosis was based upon a post-bronchodilator forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) ratio of <0.7, in the absence of a primary diagnosis of bronchiectasis, asthma or any other significant respiratory disease. The patients also had symptoms and a history compatible with COPD (disease onset after 40 years of age, smoking history of at least 10 packs/years, or occupational exposure to irritant or toxic gases or biomass exposure). Spirometric measurements were performed during stable state. The GOLD categorization of the COPD patients, whether C or D, was determined using either modified Medical Research Council (mMRC) dyspnea scale or COPD Assessment test (CAT) and severity/frequency of the exacerbations in the last 12 months. The COPD patients were not enrolled unless they met the recommended roflumilast prescription criteria (chronic bronchitis and frequent exacerbator phenotype and post-bronchodilator FEV1 less than 50% predicted on a given patient without hepatic problems) (1).
The patients were on a regular follow-up in our outpatients’ clinics. These patients were questioned and examined in a detailed and structured manner in each visit, and the data in their records were updated accordingly. The data we obtained for this study included socio-demographic information, smoking history, pulmonary function test results, AEs associated with roflumilast use, any discontinuation due to roflumilast AE and the number of COPD exacerbations in the year before the initiation of roflumilast and the number of COPD exacerbations after the initiation of roflumilast. An exacerbation was determined if a patient was prescribed antibiotics and/or systemic corticosteroids due to worsening of COPD symptoms. Hospitalizations due to respiratory causes before and after the treatment of roflumilast were also obtained. Safety assessment included all AEs reported by the physicians in patients’ medical records. The AEs leading to the drug discontinuation were also noted. AEs were defined as severe, if leading to hospitalization or death.
The statistical analysis was performed using SPSS 23.0 program. The descriptive data were presented in terms of percent and mean values with their standard deviations. The Paired Samples t-test was utilized to compare the exacerbation rates and hospitalization rates before and after the roflumilast treatment. Since patients had different follow-up periods, the annualized rates of exacerbations and hospitalizations were used in the comparison analysis. P value <0.05 was accepted to be statistically significant.
Results
Our search through the medical records yielded that 107 patients were prescribed with roflumilast therapy during the study period. There were insufficient data in the medical records of the 18 patients, and we also noted that 6 additional patients did not use the prescribed drug any time. Therefore, we could include 83 patients in the final analysis. Of the 83 patients (10 female, 73 male), 79.5% were in the GOLD D category, and 20.5% were in the GOLD C category. Eight patients (9.6%) were never smokers, and 14 patients (16.9%) were current smoker. The mean smoke load was 44.5±31.8 packs/years. The patients without smoking history had evident occupational and/or biomass exposure, and supporting their COPD diagnosis, their past medical records did not reveal any significant reversibility with neither inhaled salbutamol nor long-term COPD treatment. Their mean age was 66.2±9.3 years, mean BMI was 25.6±5.1 kg/m2, and mean duration of roflumilast use was 18.9±10.4 months. The baseline characteristics of the patients were presented in Table 1. During recruitment, all patients were already on triple regimen with ICS + LABA + LAMA.
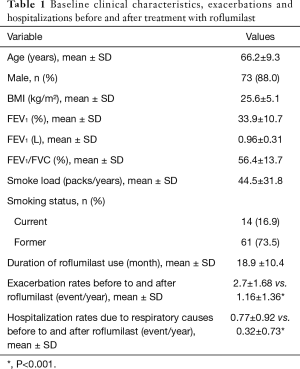
Full table
Compared to the pre-treatment period, the roflumilast use led to a significant reduction in the COPD exacerbation rates. The mean COPD exacerbation rate in the previous year was 2.7±1.68, whereas the mean number of exacerbations during roflumilast use became 1.16±1.36/year (P<0.001). Annual hospitalization rate due to respiratory causes also decreased after roflumilast treatment from 0.77±0.92 to 0.32±0.73 (P<0.001).
AEs occurred in 22 (26.5%) patients. The most frequent AEs were loss of appetite (10.8%) and weight loss (10.8%). Other AEs were nausea (8.4%), abdominal pain (7.2%), diarrhea (3.6%), myalgia (2.4%) and palpitation (1.2%). Sixteen (19.3%) patients discontinued the treatment because of AEs. None of the AEs were life-threatening (Table 2). Any discontinuation data due to the lack of efficacy were not noted in the records.
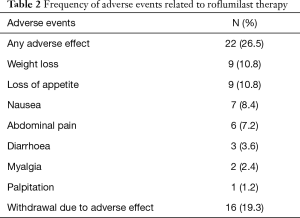
Full table
Discussion
In the present study, we showed that roflumilast decreased exacerbation and hospitalization rates in patients with severe COPD. Our real-world group also yielded an AE profile of roflumilast (26.5%) that was more prevalent than the figures reported in the RCTs and was less prevalent than the figures from previous real-world studies. In line with the previous RCTs and real-world studies, our findings also confirmed that the side effects were mainly gastrointestinal; 19.2% of our patients group discontinued the roflumilast therapy due to AEs.
Two recent meta-analysis yielded that the AE rates are approximately 10% higher in the roflumilast users compared to placebo (3,9). In the first RCT on roflumilast in 2005, the discontinuation rates in 500 and 250 µg roflumilast groups were 15.1% and 9.3%, respectively (10). In another RCT conducted by Calverley et al., the drug discontinuation rate was 13.5% in the 500 µg group (11). In two double-blind, placebo-controlled, multicenter studies done in an outpatient setting, the discontinuation rate because of AEs was 16.5% and 8.9% (12). In other two placebo-controlled, double-blind, multicentre trials the discontinuation rates were 15.5% and 13.1% (13). In a recent multicentre RCT, discontinuation rate was found 11% (14). The main reasons for discontinuation of roflumilast in the RCTs were diarrhea, nausea and headache. In real-world studies, however, reported discontinuation rates were much higher (35.1% to 84%) than these RCTs (Table 3) (5-8). Discontinuation rate in our present study was between RCTs and real-world studies (19.3%). Nevertheless, it is a very well-known phenomenon that dose titration strategies significantly reduce the discontinuation rates, and many AEs due to roflumilast may resolve or diminish during the course of the treatment (10,15).

Full table
It is not clear which variables could predict roflumilast intolerance and lead to discontinuation of treatment in clinical practice. A recent Korean study showed that the patients with low BMI were more likely to undergo drug discontinuation (BMI <23 kg/m2; OR=2.960, P=0.004) (16). But it should be noted in the study that, mean baseline BMI of Korean patients was significantly lower than that of RCTs and other real-world studies. Racial differences may lead to some differences in efficacy and safety parameters in drug responses (1). Inconsistent with the other studies, a study conducted among Chinese patients with COPD could not find any significant difference in AE rates between roflumilast and placebo groups (17). The most frequent AEs were upper respiratory tract infection (roflumilast group, 21.7% vs. placebo group, 11.7%, P=0.15) and diarrhea (roflumilast group, 13.3% vs. placebo group, 10.0%, P=0.57). A Korean study, on the other hand, showed higher percentage of AEs than placebo group (69.6% vs. 45.7%) (18). Another Korean study showed higher discontinuation rate and AEs than in previous RCTs (16). Lee et al. showed that the safety and tolerability of roflumilast in Asian patients with COPD from different cultural and ethnic backgrounds in Hong Kong, Malaysia, the Philippines, Taiwan, and South Korea were similar to that in a Caucasian population (19). To date, safety and efficacy of roflumilast have not been evaluated in Turkish population with COPD in any clinical trial. Our study also yielded that the discontinuation rate, AE rates and main AEs in Turkish patients were similar to the previous studies confirming that there was not a significant racial difference regarding the roflumilast use.
We also analyzed the effect of roflumilast on exacerbation and hospitalization rates due to COPD. Our findings were consistent with the previous studies that roflumilast reduced both COPD exacerbations and hospitalization rates. A meta-analysis including 23 trials with 19,948 participants showed that treatment with a PDE4 inhibitor was associated with a reduced likelihood of COPD exacerbation (OR 0.78, 95% CI: 0.73 to 0.83) (3). In a recent meta-analysis, when compared to placebo treatment, roflumilast significantly reduced the likelihood of COPD exacerbation by reducing the rate of hospitalization or prolonging the interval of time to exacerbation (standardized difference in mean ± SD: 0.099±0.020, 95% CI: 0.061 to 0.138; P<0.001) (4).
The present study has several limitations. This is a retrospective observational study, and sample size is relatively small due to its being conducted in a single center. However, we believe that the factors like high quality patient records with minimal missing data and quite long-term follow-up period counterbalanced these limitations. In addition to this, since the study was conducted at a university hospital, this might have led to a selection bias. However, as another counter balancing factor, the health system in Turkey allows the patients to apply any hospital directly without any limitation, leading to the homogenization of the outpatients at the hospitals. Another limitation we should mention is that we did not search for the initiation or discontinuation of other COPD drugs actively during the study period. Potentially, such changes are expected to influence the outcomes. However, the roflumilast treatment was only recommended to be prescribed as an add-on therapy in severe COPD patients already on a triple regimen (ICS/LABA/LAMA). We do not think that this baseline triple regimen was lessened or discontinued at all during the study period. For the initiation of another drug for chronic use in COPD, our health system only allows theophylline preparations for this purpose. However, the theophylline use has almost been abandoned in Turkey for the last 10 years because of its side effects, and moreover, its use is contraindicated in patients using roflumilast. Due to all these factors, we do not think that discontinuation or initiation of other COPD drugs, if any, has a significant impact on our results. We believe that the issues addressed in the present study needs to be clarified with further multicenter studies conducted in real-world settings with larger patient populations.
Conclusions
This study examined the effectiveness and safety of roflumilast therapy over an 18-month period in patients with severe COPD who had frequent exacerbations. This therapy provided a clinical benefit in these patients, with a significant reduction in exacerbations and hospitalization rates due to COPD. AE rates, although comparable to the previous studies, are common, and they led to the discontinuation of roflumilast in one-fifth of the patients. We think that since the clinical benefit of roflumilast use is clear, except the cases with severe AEs, the best effort should be done to keep the patients on the therapy with roflumilast.
Acknowledgements
The authors would like to thank to Dr. Hülya Dirol for the data collection.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Hospital’s local ethics committee and written informed consent of patients was waived.
References
- Global initiative for chronic obstructive lung disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: 2018 report. London, UK: Global Initiative for Chronic Obstructive Lung Disease, 2018. Available online: http://www.goldcopd.org
- Rabe KF, Calverley PMA, Martinez FJ, et al. Effect of roflumilast in patients with severe COPD and a history of hospitalisation. Eur Respir J 2017. [Crossref] [PubMed]
- Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2017;9:CD002309. [PubMed]
- Shen LF, Lv XD, Chen WY, et al. Effect of roflumilast on chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ir J Med Sci 2018;187:731-8. [Crossref] [PubMed]
- Muñoz-Esquerre M, Diez-Ferrer M, Montón C, et al. Roflumilast added to triple therapy in patients with severe COPD: a real life study. Pulm Pharmacol Ther 2015;30:16-21. [Crossref] [PubMed]
- Worndl E, Hunt EB, Kennedy MP, et al. Roflumilast in COPD. Chest 2015;148:e31. [Crossref] [PubMed]
- Kim KH, Kang HS, Kim JS, et al. Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2017;12:3449-56. [Crossref] [PubMed]
- Gómez-Rodríguez M, Golpe R. Intolerance to roflumilast in real-life clinical practice. Eur J Intern Med 2017;43:e28-9. [Crossref] [PubMed]
- Rogliani P, Calzetta L, Cazzola M, et al. Drug safety evaluation of roflumilast for the treatment of COPD: a meta-analysis. Expert Opin Drug Saf 2016;15:1133-46. [Crossref] [PubMed]
- Rabe KF, Bateman ED, O’Donnell D, et al. TD. Roflumilast–an oral anti-inflammatory treatment for chronic obstructive pulmonary disease: a randomised controlled trial. Lancet 2005;366:563-71. [Crossref] [PubMed]
- Calverley PM, Sanchez-Toril F, McIvor A, et al. Effect of 1-year treatment with roflumilast in severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2007;176:154-61. [Crossref] [PubMed]
- Calverley PM, Rabe KF, Goehring UM, et al. M2-124 and M2-125 study groups. Roflumilast in symptomatic chronic obstructive pulmonary disease: two randomized clinical trials. Lancet 2009;374:685-94. [Crossref] [PubMed]
- Fabbri LM, Calverley PM, Izquierdo-Alonso JL, et al. M2-127 and M2-128 study groups. Roflumilast in moderate-to-severe chronic obstructive pulmonary disease treated with longacting bronchodilators: two randomized clinical trials. Lancet 2009;374:695-703. [Crossref] [PubMed]
- Martinez FJ, Calverley PM, Goehring UM, et al. Effect of roflumilast on exacerbations in patients with severe chronic obstructive pulmonary disease uncontrolled by combination therapy (REACT): a multicentre randomised controlled trial. Lancet 2015;385:857-66. [Crossref] [PubMed]
- Watz H, Bagul N, Rabe KF, et al. Use of a 4-week up-titration regimen of roflumilast in patients with severe COPD. Int J Chron Obstruct Pulmon Dis 2018;13:813-22. [Crossref] [PubMed]
- Kim KH, Kang HS, Kim JS, et al. Risk factors for the discontinuation of roflumilast in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis 2017;12:3449-56. [Crossref] [PubMed]
- Liu DY, Wang ZG, Gao Y, et al. Effect and safety of roflumilast for chronic obstructive pulmonary disease in Chinese patients. Medicine (Baltimore) 2018;97:e9864. [Crossref] [PubMed]
- Lee JS, Hong YK, Park TS, et al. Efficacy and Safety of Roflumilast in Korean Patients with COPD. Yonsei Med J 2016;57:928-35. [Crossref] [PubMed]
- Lee SD, Hui DS, Mahayiddin AA, et al. Roflumilast in Asian patients with COPD: A randomized placebo-controlled trial. Respirology 2011;16:1249-57. [Crossref] [PubMed]


