
The role of extracorporeal life support in the management with severe idiopathic pulmonary artery hypertension undergoing lung transplantation: are those patients referred too late?
Introduction
Idiopathic pulmonary artery hypertension (iPAH) is a clinical condition characterised by pre-capillary pulmonary hypertension (i.e., pulmonary capillary wedge pressure ≤15 mmHg) at right heart catheterisation. iPAH may be idiopathic, heritable, induced by drug or toxin exposure, or develop as a consequence of different disorders, such as connective tissue diseases, HIV infection, congenital heart diseases or portal hypertension (1). The estimated prevalence of iPAH is ≤15–50 patients per one million of the population (2,3). According to the National Institutes of Health (NIH) in the United States, pulmonary hypertension was associated with an overall survival for iPAH patients of 2.8 years (4). Conventional management of iPAH, apart from general measures to help alleviate symptoms, include oral anticoagulants, diuretics and oxygen; while the targeted therapies that developed in in recent decades act on prostacyclin, endothelin, and nitric oxide pathways to improve endothelial dysfunction (5,6). Patients who fail to improve or show clinical worsening despite maximal medical therapy, have a very poor prognosis and should routinely be assessed for lung transplantation (LTx) (5). iPAH forms one of the less common indications for LTx comprising 3% of all indications (7). It has already been shown that increasing pulmonary artery pressure, while awaiting LTx, is associated with worse long-term survival following LTx (8). Primary graft dysfunction (PGD) is the main cause of early morbidity and mortality after LTx and iPAH is one of the major risk factors for PGD (9). Extracorporeal life support (ECLS) is rarely utilized in patients with severe iPAH as a bridge to LTx or as a rescue in PGD following LTx for iPAH while being the only possible rescue therapy for this demanding patient cohort. In this respect, we evaluated the utility of ECLS in the management of severe iPAH for a patient cohort undergoing LTx in our institution.
Methods
The Institutional Review Board at our center approved this study and waived the need for individual patient consent. The study design was a retrospective review of the prospectively collected data. A total of 321 LTx were performed at our institution between January 2007 and May 2014, whereas, 15 LTx were performed for iPAH as a cause of end-stage lung disease.
Endpoints of the study
Primary endpoints of the study were overall survival after LTx and bronchiolitis obliterans syndrome (BOS) free survival. Secondary end points included early postoperative recipient characteristics, such as paO2/FiO2 ratio at the end of the transplant, 24, 48 and 72 h after transplant, duration of mechanical ventilation, ICU and total hospital stay, as well as the need for postoperative use of ECLS.
Organ assessment and organ procurement protocol
Organ procurement was performed by six designated heart and lung transplant centers in the United Kingdom, including ours, within specified geographical region of each center. The lungs were matched to the recipients according to blood group, height, total lung capacity, time already spent on the LTx waiting list, and the clinical status of the recipient at the time of the transplantation. Donor organ assessment performed at the donor hospitals included radiological assessment, fibre optic bronchoscopy, gross organ inspection and palpation, assessment of compliance using deflation test and selective blood-gas analysis from each pulmonary vein. The preservation solution used was low potassium dextran (Perfadex, Medisan, Uppsala, Sweden) solution augmented with CaCl2, 3.6% tromethamine (THAM, Hospira Inc., Lake Forest, IL, USA), and epoprostenol sodium 2.5 mL/L. The total ischemic time was defined as the time between cardiac arrest in donors after cardiac death (DCD) or as the aortic cross clamp time in donors after brain death (DBD) and the reperfusion of the second implanted lung.
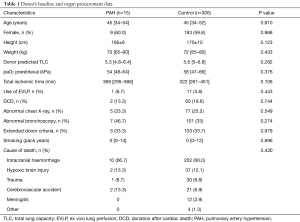
Detailed donor data, such as demographic parameters, cause of death, current clinical status, laboratory investigations, and past social and medical history, were analyzed. Demographics and perioperative recipient data as well as mid-term outcomes were compared. BOS was diagnosed when, post-transplant, fraction of expired volume in 1 second (FEV1), measured on a regular basis, permanently dropped >20% of the best FEV1 achieved after the LTx.
Types of ECLS
Veno-venous (VV) extracorporeal membrane oxygenation (ECMO) is a most commonly used ECLS as a bridge to LTx. A dual-lumen single cannula system enables VV ECMO via cannulation of only the internal jugular vein, allowing the patient to ambulate and rehabilitate, thus decreasing the risk of ventilator-associated pneumonia and deconditioning. In some patients, because of refractory hypoxemia, severe pulmonary hypertension, and right ventricular dysfunction refractory to inhaled nitric oxide and milrinone therapy, veno-arterial (VA) ECMO is chosen over VV to unload right ventricle and to prevent shunting through the pulmonary vasculature. Extra-corporeal CO2 removal systems, such as interventional lung assist (iLA) membrane ventilator (Novalung GmbH, Heilbronn, Germany) are pumpless extracorporeal lung-assist devices, which can be implanted via peripheral (femoral artery to femoral vein) or central (left pulmonary artery to left atrium) access. Not only it is less traumatic for blood cells due to the lacking pump in the circuit, it is effective for clearance of carbon dioxide with minimal oxygenation. Haemolung respiratory assist system (RAS) (ALung Technologies Inc., PA, USA) combines a centrifugal blood-pump, a gas-exchange membrane and a single VV (femoral/jugular) dual-lumen 15.5 Fr catheter.
Statistical analysis
All data were analyzed using IBM SPSS Statistics for Windows, Version 21 (IBM Corp., Released 2012. Armonk, NY: IBM Corp) and are presented as continuous or categorical variables. Continuous data were evaluated for normality using one sample Kolmogorov-Smirnov-test and confirmed by histograms. Continuous variables were expressed as the mean ± standard deviation in cases of normal distributed variables or median (interquartile range) in cases of non-normal distributed variables. Categorical variables are presented as total numbers of patients and percentages. Continuous data were analyzed with unpaired t-test for normally distributed variables and Mann Whitney U-test for non-normally distributed variables. Pearson’s χ2 or Fisher exact tests were used for categorical data dependent on the minimum expected count in each crosstab. Kaplan-Meier survival estimation was applied for survival analysis of the entire patient cohort. Log rank (Mantel-Cox) test was applied for comparison of cumulative survival and BOS free survival estimates of patients from hanging and control group. P values <0.05 were considered statistically significant. Propensity score matching function of SPSS software was conducted to reduce confounding bias between the groups. A propensity score for each patient was estimated using a logistic regression model with preoperative characteristics that showed statistically significant differences between the two groups as independent variables. Matching was based on one-to-three nearest neighbor matching method with a tolerance level on the maximum propensity score distance (calipers of width 0.2 standard deviations of the logit of the PS). This propensity score based matching procedure resulted in a total number of 60 patients who were well matched for baseline characteristics.
Results
Among 321 LTx, 296 (92%) were double-lung (DLTx) and 25 (8%) were single-LTx (SLTx). In 60 (18.7%) cases lungs were retrieved from donators after cardiac death (DCD-donors).
Idiopathic pulmonary hypertension cohort
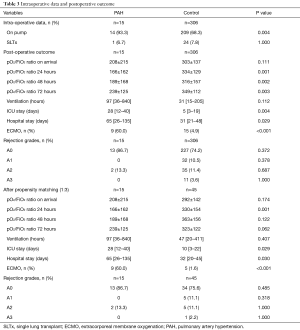
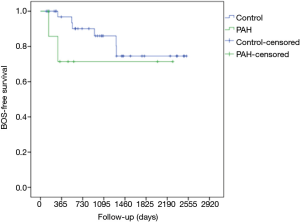
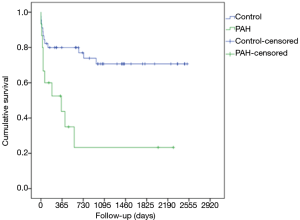
Among 15 LTx in iPAH patients, 13 were DLTx and 2 were SLTx. The most frequent donor cause of death was intracranial hemorrhage (66.7%), followed by hypoxic brain injury (13.3%), cerebrovascular accident (13.3%) and trauma (6.7%). In 2 cases lungs were retrieved from the DCD donors, whereas one pair of donor lungs were assessed and resuscitated on ex vivo lung perfusion (EVLP) system. A significant proportion of donor lungs met extended criteria (33.3%), were associated with abnormal chest X-ray (33.3%) or abnormal bronchoscopy (46.7%). The median total ischemic time for this cohort was 369 (range, 295–588) min. The median recipient’s age was 42 (range, 40–49) years whereas 47% of patients were female. The demographics and preoperative baseline characteristics of the donors and the recipients in detail are given in Tables 1 and 2, respectively. Intraoperative variables and parameters of early postoperative outcome are presented in Table 3. Most patients (93.3%) required on-pump LTx and one patient received single lung transplant. Mean pO2/FiO2 ratios directly after transplant as well as at 24, 48 and 72 hours after surgery accounted for 208±215, 166±162, 189±168, and 239±125 mmHg, whereas 60% of patients required VA ECMO support for PGD. Median ICU and hospital stay were 28 (range, 12–40) and 65 (range, 26–135) days, respectively. Freedom from BOS at 1, 3, and 5 years was 75%, 50%, and 50% (Figure 1), whereas overall cumulative survival at 1, 3, and 5 years accounted for 57.8%, 39.6% und 19.8% (Figure 2).
Full table

Full table
Full table
Utilization of ECLS in patients with iPAH
Four patients in iPAH group were bridged to LTx. Two patients were bridged with extra-corporeal CO2 removal utilizing central iLA membrane ventilator (Novalung GmbH, Heilbronn, Germany) while 2 patients were bridged with VA ECMO utilizing CentriMag® (Thoratec; CA, USA) centrifugal pump and Medos Hilite® 7000LT (Medos®, Germany) oxygenator. All patients survived 30 days after transplant, whereas 3 patients were alive at the cut-off of the study. One patient died on day 38 after transplantation due to bowel ischemia leading to multi-organ failure. Eight patients required ECLS after LTx for primary graft failure. In all cases a VA ECMO was used for support. One patient died on day 13 after transplantation, 3 patients survived 30 days and less than one year, while 4 patients survived more than one year after transplantation. Multi-organ failure was the cause of death in all non-survivors.
Unadjusted univariate analysis
The demographics and preoperative baseline characteristics of the donors and the recipients in iPAH group and control group are given in Table 1 and 2, respectively. The groups were comparable in these terms except for requirement for ECLS in recipients as a bridge to LTx that was significantly higher in iPAH group compared to the control group (27% vs. 3%). Intraoperative variables and parameters of early postoperative outcome are presented in Table 3. A significantly higher number of patients in PAH group required on-pump LTx and they had significantly lower PO2/FiO2 ratio at 24, 48 and 72 hours after LTx. More patients in PAH group required post-LTx ECLS and had significantly longer ICU and hospital stay. The incidence of postoperative BOS and rejection was comparable between the groups whereas mid-term survival in iPAH group was statistically poorer compared to the control group (log rank, Generalized Wilcoxon and Tarone-Ware P<0.001, respectively) accounting for 43.8% vs. 86.6%, 23.3% vs. 74.3% and 23.3% vs. 64.4% at 1, 3 and 6 years after LTx, respectively.
Results after propensity score matching
To minimize the potential effects of selection bias on patient characteristics, we performed an additional analysis using one-to-three propensity score matching. The matching was performed based on donor and recipient characteristics that were statistically different between the two groups in the analysis of the entire cohort and further clinically important variables, such as donor gender, age, type of donation (DBD/DCD), pre-retrieval pO2/FiO2-ratio, quality of chest X-ray and bronchoscopy, utilization of EVLP, extended donor criteria, donor smoking history quantified as pack-years as well as recipient gender, age and SLTx or DLTx transplant. These two propensity-matched groups became well balanced, and no significant differences were observed in donor and recipient baseline characteristics.
After propensity score matching, there were still statistically significant differences in pO2/FiO2 ratio at 24 h postoperatively, length of ICU and hospital stay and need for postoperative ECMO, in favor of the control group (Table 3). Overall cumulative survival was still significantly poorer in the iPAH group (log-rank P=0.002), whereas there were no statistically significant differences in terms of freedom from BOS (log-rank P=0.291) (Figures 1 and 2).
Discussion
Principle finding in this study was that iPAH patients referred for LTx are referred at late stage of the disease requiring considerable pre LTx support leading to poor post LTx outcomes.
The importance of early referral of iPAH patients for LTx
The advent of disease-specific therapy for severe iPAH has reduced patient referral for lung transplant programs. Although, both heart-lung and DLTx have been performed for iPAH, due to the shortage of donor organs, most patients are considered for double LTx. In a recent report describing the impact of the lung allocation score on patients with iPAH, Chen and colleagues found that the risk of death while on the waiting list was the highest for patients with iPAH compared to other indications for LTx (10). Therefore, it is important to emphasize that patients who fail to improve or show clinical worsening despite maximal medical therapy are associated with a very poor prognosis and should routinely be assessed for LTx (5). Patients referred for transplantation before irreversible pulmonary and overall deterioration may not require extreme preoperative measures, such as ECLS and may have better survival after transplantation.
ECLS as a bridge to LTx
Bridging patients to LTx using ECLS represents maximal therapy for severe respiratory failure. The Toronto lung transplant program, while evaluating their results between 1997 and 2010, found that the aggressive management with ECLS in iPAH patients awaiting LTx could have a major impact on reducing waiting list mortality; however, the rate of severe PGD, 30-day mortality rate, and long-term outcomes remained similar. Moreover, post-transplant ICU and hospital stay increased with ECLS as a bridge to LTx (11). In the present study, one fourth of patients with iPAH were successfully bridged to LTx with one death after LTx due to bowel ischemia.
Awake ECLS bridge
Percentage of recipients requiring ventilator support before LTx increased from 2.7% in 2001 to 7.4% In the United States in 2011 (12). Traditionally, ECMO patients are heavily sedated to prevent inadvertent cannula dislodgement and to avoid respiratory compromise, which makes mechanical ventilation mandatory. This in turn leads to an increased risk of complications associated with immobility, prolonged ventilation and enteral feeding. Series of patients with end stage lung disease treated with ECMO while remaining awake have recently been published (13,14). Data by Fuehner et al. demonstrated improved survival in patients bridged to LTx using awake ECMO strategy when compared to those managed with conventional mechanical ventilation, showing potential advantages of minimizing sedation (13). In the present study we maintained all 4 patients awake while on ECLS until LTx. The key benefit of maintaining patients awake or awakening patients on ECMO therapy is the avoidance of complications associated with general anaesthesia, intubation and mechanical ventilation (15). As patients remain awake with restrictive mobility, the complications associated with chronic immobility are avoided. They are subjected to active physiotherapy including breathing as well as limb exercises helping them keeping active and preventing pressure sores, muscle loss, joint stiffness and softening of bones.
Survival in patients with iPAH bridged to LTx using ECLS
Among lung transplant recipients, those treated for iPAH have the lowest survival rates (16,17). Unadjusted 3-month mortality remains the highest with iPAH (23%) compared to other indications for LTx as reported in 29th adult lung and heart-lung transplant report published in 2012 (18). In the present study, 1-year survival was 43.8% and 6-year survival was 23.3%. The prominent reason for high mortality in our cohort may be higher incidence of preoperative invasive mechanical ventilation and ECLS.
ECMOs, hospitalization at time of transplant and oxygen dependence remain the strongest negative predictors for one-year survival in United Network for Organ Sharing (UNOS) data (19). UNOS data [1987–2008] showed unadjusted survival of patients after LTx with pre LTx mechanical ventilation and ECMO being 62% and 50%, respectively, compared to 79% in unsupported patients (20). We do believe that all patients started on ECLS would have died before their transplant if they could not have been stabilized by ECLS. Optimization of failing right ventricle in severe iPAH with ECLS may help in early post LTx period and our views are shared by others (11).
Primary graft failure in iPAH and the role of ECLS
PGD is a life threatening complication after LTx with an incidence up to 25% (21). No specific treatments have been shown to be effective and management is mainly supportive. Pulmonary artery hypertension is quoted as one of the significant and independent risk factors for PGD after LTx (9,22). In our recently published work on risk factors predictive of one-year mortality after LTx, we found that the need for post LTx ECLS was an independent predictor (23). While the right ventricle afterload is immediately reduced after DLTx, right ventricular systolic and left ventricular diastolic functions do not improve immediately leading to potential haemodynamic instability in the early post LTx period in patients with iPAH. ECLS, particularly VA ECMO reduces preload, improves systemic circulation and helps in terms of efficient gas exchange. This not only allows the right and left ventricle to adapt to the new lungs with less pulmonary vascular resistance, but also protects the lungs from flooding with the hyperdynamic right ventricle. The ECMO can be weaned over 3 to 7 days depending upon the hemodynamic and ventilation status on ECLS.
Limitations
This study is a retrospective analysis of prospectively collected data from a single institution. Although data collection in a single centre does not often encompass variability in data entry, some grade of inconsistency and missing data could not be ruled out. Also, since this was an observational study, there were no specific measures or protocols to randomize patients into any groups. The study deals with the idiopathic variety of PAH; it does not analyze secondary PAH or other types of pulmonary hypertension as an etiology of end-stage lung disease.
Conclusions
ECLS as a bridge to LTx is a boon for iPAH patients with rapid deterioration in lung and right ventricular function. Awakening patients on ECLS should be attempted if possible. Despite utilization of ECLS in the management of iPAH, the outcomes in terms of PGD and survival remain poor. iPAH patients with end stage lung disease should be referred for LTx assessment at early stage and a higher lung allocation score should be attributed to iPAH patients on waiting lists so that they receive LTx before it is too late.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The Institutional Review Board at our center approved this study and waived the need for individual patient consent.
References
- O'Callaghan DS, Humbert M. A critical analysis of survival in pulmonary arterial hypertension. Eur Respir Rev 2012;21:218-22. [Crossref] [PubMed]
- Humbert M, Sitbon O, Chaouat A, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006;173:1023-30. [Crossref] [PubMed]
- Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007;30:104-9. [Crossref] [PubMed]
- D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991;115:343-9. [Crossref] [PubMed]
- Task Force for Diagnosis and Treatment of Pulmonary Hypertension of European Society of Cardiology (ESC). European Respiratory Society (ERS). Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219-63. [Crossref] [PubMed]
- O'Callaghan DS, Savale L, Montani D, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nat Rev Cardiol 2011;8:526-38. [Crossref] [PubMed]
- Gottlieb J. Lung transplantation for interstitial lung diseases and pulmonary hypertension. Semin Respir Crit Care Med 2013;34:281-7. [Crossref] [PubMed]
- Ohtsuka T, Flaherty KR, Lin J, et al. Preoperative pulmonary artery pressure and mortality after lung transplantation. Asian Cardiovasc Thorac Ann 2013;21:326-30. [Crossref] [PubMed]
- Diamond JM, Lee JC, Kawut SM, et al. Clinical risk factors for primary graft dysfunction after lung transplantation. Am J Respir Crit Care Med 2013;187:527-34. [Crossref] [PubMed]
- Chen H, Shiboski SC, Golden JA, et al. Impact of the lung allocation score on lung transplantation for pulmonary arterial hypertension. Am J Respir Crit Care Med 2009.468-74. [Crossref] [PubMed]
- de Perrot M, Granton JT, McRae K, et al. Impact of extracorporeal life support on outcome in patients with idiopathic pulmonary arterial hypertension awaiting lung transplantation. J Heart Lung Transplant 2011;30:997-1002. [Crossref] [PubMed]
- Organ Procurement and Transplantation Network Data Reports. Available online: http://optn.transplant.hrsa.gov/data/, accessed Feb 9, 2014.
- Fuehner T, Kuehn C, Hadem J, et al. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med 2012;185:763-8. [Crossref] [PubMed]
- Olsson KM, Simon A, Strueber M, et al. Extracorporeal membrane oxygenation in nonintubated patients as bridge to lung transplantation. Am J Transplant 2010;10:2173-8. [Crossref] [PubMed]
- Hess DR. Approaches to conventional mechanical ventilation of the patient with acute respiratory distress syndrome. Respir Care 2011;56:1555-72. [Crossref] [PubMed]
- Trulock EP. Lung transplantation for primary pulmonary hypertension. Clin Chest Med 2001;22:583-93. [Crossref] [PubMed]
- Christie JD, Edwards LB, Aurora P, et al. The registry of the international society for heart and lung transplantation: twenty-sixth official adult lung and heart-lung transplantation Report-2009. J Heart Lung Transplant 2009;28:1031-49. [Crossref] [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant 2012;31:1073-86. [Crossref] [PubMed]
- Russo MJ, Davies RR, Hong KN, et al. Who is the high-risk recipient? Predicting mortality after lung transplantation using pretransplant risk factors. J Thorac Cardiovasc Surg 2009;138:1234-8.e1. [Crossref] [PubMed]
- Mason DP, Thuita L, Nowicki ER, et al. Should lung transplantation be performed for patients on mechanical respiratory support? The US experience. J Thorac Cardiovasc Surg 2010;139:765-73.e1. [Crossref] [PubMed]
- Boffini M, Ranieri VM, Rinaldi M. Lung transplantation: is it still an experimental procedure? Curr Opin Crit Care 2010;16:53-61. [Crossref] [PubMed]
- Kuntz CL, Hadjiliadis D, Ahya VN, et al. Risk factors for early primary graft dysfunction after lung transplantation: a registry study. Clin Transplant 2009;23:819-30. [Crossref] [PubMed]
- Sabashnikov A, Weymann A, Mohite PN, et al. Risk factors predictive of one-year mortality after lung transplantation. Eur J Cardiothorac Surg 2014;46:e82-8. [Crossref] [PubMed]


