Early β-blockers administration might be associated with a reduced risk of contrast-induced acute kidney injury in patients with acute myocardial infarction
Introduction
Contrast-induced acute kidney injury (CI-AKI) is a frequent complication following contrast medium (CM) exposure, which prolongs hospitalization, increases mortality and costs of health-care (1). In patients with acute myocardial infarction (AMI), the incidence of CI-AKI ranges from 10% to 30% during the hospitalization, and patients with AMI who suffer cardiogenic shock will exceed 50% (2-6). Among patients with AMI, compared with patients who did not had CI-AKI, those who had CI-AKI would increase fivefold short-term mortality and about sevenfold long-term mortality (7).
There is definitive evidence showing that patients with AMI or heart failure had higher risk of suffering acute renal damage because of sympathetic activation (8). ACCF/AHA Guidelines recommended that β-blockers should be used in patients with AMI or heart failure because of its long-term prognosis improvement (9-11). β-blockers can reduce sympathetic activation that decrease the risk of acute renal damage. However, it’s still not clear whether β-blockers can reduce the CI-AKI rates among patients with AMI who are undergoing coronary angiography (CAG) or percutaneous coronary intervention (PCI).
Methods
Study population
This is a single centre, prospective observational study (PROCOMIN, ClinicalTrials.gov NCT01400295). From January 2010 to December 2013, 1,312 patients with AMI who were undergoing CAG or PCI in Guangdong Provincial People’s Hospital were recruited in the study. Three patients were excluded because of end-stage renal disease (eGFR <15 mL/min/1.73 m2). At last, 1,309 patients were included in the subsequent analysis. Selective operation patients received a continuous hydration therapy of isotonic saline at a speed of 0.5–1 mL/kg/h for at least 2–12 hours before and continuing 6–24 hours after the procedure. Emergency operation patients received unlimited hydration intervention before procedure. All patients’ serum creatinine (SCr) were tested since admission and till 3 days after the procedure. The study was approved by the ethics committee of our institution, and all patients have signed an agreement on informed consent.
Clinical definitions and follow-up
The operational definitions used to describe the epidemiology of CI-AKI is shown in Table 1. We obtained patients’ follow-up events through office visits and telephone interviews at 1, 6, 12, 24, 36, and 48 months after enrollment. The mean follow-up time was 2.35±0.99 years

Full table
Statistical analysis
Patients were divided into the β-blockers group and the non β-blockers group. For continuous variables, 2 independent samples t-tests were conducted for normally distributed data [expressed as mean ± standard deviation (SD)] and the Wilcoxon rank-sum test was used for non-normal distribution (presented as median and IQR). Pearson’s chi-square test or Fisher’s exact test was used for categorical data, which were expressed as percentages. Univariate analysis and multiple logistic regression models with stepwise selection and backward elimination were used to identify predictors of rates of CI-AKI for AMI patients who underwent CAG or PCI with β-blockers. Kaplan-Meier analysis was used to count the cumulative mortality, and log-rank test was used to assess differences between curves. Multivariate Cox regression analysis was used to calculate the hazard ratios (HRs) and their 95% confidence intervals (CIs). All data analyses were performed using IBM PASW-SPSS Statistics 22.0 statistical software package (SPSS Inc., Chicago, IL, USA) and R software (version 3.1.2, R Core Team, Vienna, Austria) (16). Two-sided P values <0.05 were considered statistically significant.
Results
Baseline characteristics
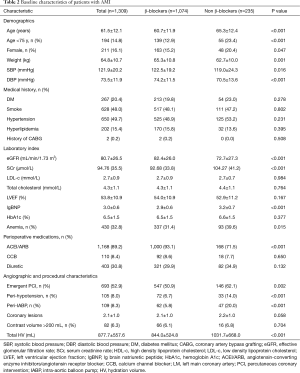
Overall, 1,309 AMI patients who were undergoing CAG or PCI were included in the final analysis. Among them, 16.1% of patients were females, mean age was 61.5±12.1 years, mean SCr level was 94.76±35.51 mg/dL, and left ventricular ejection fraction (LVEF) was 53.8%±10.9% at baseline (Table 2). Compared with patients in the β-blockers group (n=1,074), patients in the non-β-blockers group (n=235) were older (P<0.001) and with lighter weight (P<0.001), worse renal function (P<0.001), higher rate of peri-hypotension (P<0.001), more usage of intra-aortic balloon pump (IABP) (P<0.001).
Full table
Primary and secondary endpoint
During the study period, the rates of CI-AKI was higher in non β-blockers group (β-blockers group vs. non β-blockers group =17.5% vs. 25.1% respectively; P<0.007). When analyzing the short and long prognosis as a categorical variable, using β-blockers was associated with a significant reduction in cardiovascular events, including death and MACE (cardiovascular death, non-fatal myocardial infarction or non-fatal stroke) (Table 3).

Full table
Association of β-blockers with CI-AKI
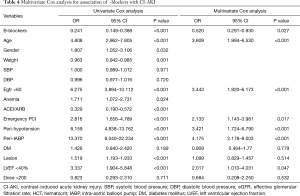
This paper used univariate analysis logistic regression to do stepwise selection to identify predictors of CI-AKI for AMI patients who underwent CAG or PCI. After multivariate logistic regression analysis adjusting, 10 confounders were recognized to have an association with CI-AKI, including β-blockers [β-blockers vs. non β-blockers, odds ratio (OR) =0.520; 95% CI, 0.291–0.930; P=0.027], age, diabetes mellitus (DM), eGFR <60 mL/min/1.73 m2, LVEF <40%, use of IABP, peri-hypotension, emergent PCI, coronary lesions, and CM dose >200 mL (Table 4).
Full table
All-cause mortality during the follow-up
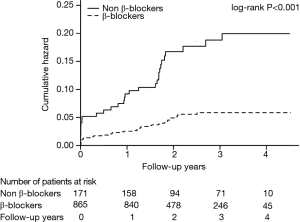
The mean follow-up period was 2.35±0.99 years. Figure 1 shows the Kaplan-Meier survival curves. Compared with the β-blockers group, cumulative mortality of patients in the non-β-blockers group was significantly higher (log-rank P<0.001).
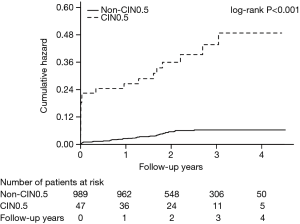
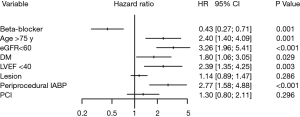
In addition, multivariate Cox regression analysis indicated that not taking β-blockers was associated with a significantly increased risk of death after PCI [β-blockers vs. non-β-blockers: HR =0.43; 95% CI, 0.27–0.71; P=0.001; Figure 2]. Moreover, during the follow-up, patients who developed CI-AKI had a higher rate of all-cause mortality than those who did not (Figure 3).
Discussion
It’s the first time to show that AMI patients who were undergoing CAG or PCI may get benefit from taking β-blockers within 24 hours of perioperative period in decreasing the risk of CI-AKI. During the study period, taking β-blockers was associated with short- and long- prognosis improvement.
Recently, a few studies reported that patients with AMI would have a high-risk of developing CI-AKI, ranging from 10% to 30% during hospitalization (1-4). After multivariate regression adjusted, some risk factors are associated with developing CI-AKI [previous history of DM or CKD) (17), procedure information (CM dose, CABG), and cardiac complications (hemodynamic instability, heart failure)]. The physiopathology of CI-AKI is not clear. At present, the physiopathology of CI-AKI tends to concentrate on CM induced toxic damage to renal tubular epithelial cells, and renal perfusion decrease would aggravate the toxic injury. Cardiac output decrease and excessive activation of the sympathetic nerve are important reasons for reduction of renal perfusion. A recent hypothesis more strongly supports the association of AMI with sympathetic hyperactivity, high heart rate of patients with AMI is closely related to the occurrence of acute renal injury (18,19).
The 2014 AHA/ACC guideline clearly pointed out that patients with AMI should take β-blockers except for those patients who are complicated with contraindications such as a low heart rate, acute decompensated heart failure, or bronchoconstriction (20,21). Hirschl et al. reports that patients with ST-segment elevation myocardial infarction (STEMI) may also get benefit from the early use of β-blockers (22). β-blockers improve endothelial dysfunction in renal ischemia because of endothelial nitric oxide synthase activation (23). Most studies indicated that hemodynamic activation would play a significant role in the worsened renal function, active sympathetic nervous and renin-angiotensin-aldosterone system (24). Finally, β-blockers can interdict the vicious circle by reducing the sympathetic nerve system activity that improve the prognosis (8).
After multivariate adjusting, 10 variables were associated with CI-AKI, 3 (peri-hypotension, LVEF <40%, and IABP) are directly reflected in worsened cardiac function and poor prognosis. In conclusion, early β-blockers administration may keep renal function and reduce mortality by decreasing sympathetic activity in patients with AMI who are undergoing CAG or PCI.
Limitations
This study has several limitations. This study is an observational, single-centre study. The evidence may not be as strong as that of a randomized, controlled trial. Besides, though we have calculated the hydration volumes, data of water intake hydration volumes is lacked.
Conclusions
Our study shows that early β-blockers administration might be associated with a moderately reduced risk of CI-AKI and long-term mortality among patients with AMI who are undergoing CAG or PCI. This needs to be further investigated and validated in further large-scale, multi-centre, randomized, controlled trials.
Acknowledgements
We appreciate the efforts of our statistical consultant, An Fan, MD, Guangzhou 510515, Guangdong, China.
Funding: The study was supported by grants from the Guangdong General Hospital Clinical Research Fund (2014dzx02) and Science and Technology Planning Project of Guangdong Province (2014B070706010) as major funding bodies. This study was also supported by the Progress of Science and Technology Project in Guangdong Province (grant numbers: 2013b031800025, 2016b020215130, 2017B020247060), Cardiovascular Research Foundation Project of Chinese Medical Doctor Association (SCRFCMDA201216) and Beijing Lisheng Cardiovascular pilot Foundation (LHJJ201612127).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: Ethics approval has been obtained from the institutional research ethics boards at Guangdong General Hospital Ethics Research Committee and all other participating sites. Written informed consent was obtained from all participants.
References
- Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol 2004;44:1780-5. [Crossref] [PubMed]
- Fox CS, Muntner P, Chen AY, et al. Short-term outcomes of acute myocardial infarction in patients with acute kidney injury: a report from the national cardiovascular data registry. Circulation 2012;125:497-504. [Crossref] [PubMed]
- Centola M, Lucreziotti S, Salerno-Uriarte D, et al. A comparison between two different definitions of contrast-induced acute kidney injury in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Int J Cardiol 2016;210:4-9. [Crossref] [PubMed]
- Parikh CR, Coca SG, Wang Y, et al. Long term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 2008;168:987-95. [Crossref] [PubMed]
- Hwang SH, Jeong MH, Ahmed K, et al. Different clinical outcomes of acute kidney injury according to acute kidney injury network criteria in patients between ST elevation and non-ST elevation myocardial infarction. Int J Cardiol 2011;150:99-101. [Crossref] [PubMed]
- Marenzi G, Assanelli E, Campodonico J, et al. Acute kidney injury in ST-segment elevation acute myocardial infarction complicated by cardiogenic shock at admission. Crit Care Med 2010;38:438-44. [Crossref] [PubMed]
- Goldberg A, Hammerman H, Petcherski S, et al. In hospital and 1-year mortality of patients who develop worsening renal function following acute ST-elevation myocardial infarction. Am Heart J 2005;150:330-7. [Crossref] [PubMed]
- Fujii T, Kurata H, Takaoka M, et al. The role of renal sympathetic nervous system in the pathogenesis of ischemic acute renal failure. Eur J Pharmacol 2003;481:241-8. [Crossref] [PubMed]
- Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003;107:1570-5. [Crossref] [PubMed]
- Yancy CW, Jessup M, Bozkurt B, et al. ACCF/AHA Guideline for the management of heart failure. Circulation 2013;128:e240-327. [PubMed]
- Rydén L, Ariniego R, Arnman K, et al. A double-blind trial of metoprolol in acute myocardial infarction. Effects on ventricular tachyarrhythmias. N Engl J Med 1983;308:614-8. [Crossref] [PubMed]
- Nutritional anemias: report of a WHO Scientific Group. Geneva: World Health Organization, 1968.
- Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137-47. [Crossref] [PubMed]
- Manjunath G, Tighiouart H, Ibrahim H, et al. Level of kidney function as a risk factor for atherosclerotic cardiovascular outcomes in the community. J Am Coll Cardiol 2003;41:47-55. [Crossref] [PubMed]
- Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention. J Am Coll Cardiol 2004;44:1393-9. [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available online: http://www.r-project.org/. Accessed July 1, 2015.
- Shaw A. Models of preventable disease: contrast-induced nephropathy and cardiac surgery-associated acute kidney injury. Contrib Nephrol 2011;174:156-62. [Crossref] [PubMed]
- Queiroz RE, de Oliveira LS, de Albuquerque CA, et al. Acute kidney injury risk in patients with ST-segment elevation myocardial infarction at presentation to the ED. Am J Emerg Med 2012;30:1921-7. [Crossref] [PubMed]
- Parikh CR, Coca SG, Wang Y, et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med 2008;168:987-95. [Crossref] [PubMed]
- Amsterdam EA, Wenger NK, Brindis RG, et al. 2014 AHA/ACC Guideline for the Management of Patients with Non-ST-Elevation Acute Coronary Syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139-228. [Crossref] [PubMed]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;61:e78-140. [Crossref] [PubMed]
- Hirschl MM, Wollmann CG, Erhart F, et al. Benefit of Immediate Beta-Blocker Therapy on Mortality in Patients With ST-Segment Elevation Myocardial Infarction. Crit Care Med 2013;41:1396-404. [Crossref] [PubMed]
- Feng MG, Prieto MC, Navar LG. Nebivolol-induced vasodilation of renal afferent arterioles involves β3-adrenergic receptor and nitric oxide synthase activation. Am J Physiol Renal Physiol 2012;303:F775-82. [Crossref] [PubMed]
- Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol 2016;32:247-55. [Crossref] [PubMed]