Fat-free mass index is superior to body mass index as a novel risk factor for prolonged air leak complicating video-assisted thoracoscopic surgery lobectomy for non-small-cell lung cancer
Introduction
Rationale
As one of the most frequent complications after pulmonary resections, prolonged air leak (PAL) remains a bothersome problem for thoracic surgeons in their daily practice and draws substantial attention in the thoracic surgery specialty (1-3). In general, 30–50% of patients who undergo lobectomy detect an air leak from the chest drainage system either immediately after operation or on postoperative day (POD) 1 (1,2). Although the presence of air leak progressively ceases several days later, approximately 8–15% of these air leak cases will ultimately develop a PAL, defined by current convention as an air leak that persists beyond 5 PODs, resulting in a dramatically increased rate of adverse morbidity and consequently a higher healthcare cost correlated with more frequent inpatient and outpatient resource utilization (1-3). Therefore, a better understanding of the predisposing factors for PAL will be extremely crucial to assist in adopting a series of prophylactic strategies to prevent the occurrence of this complication (2).
As the most common surrogate measure for obesity in current practice, body mass index (BMI) has been reported to serve as a significant risk factor for PAL in recent large-scale registry-studies (4,5). However, BMI may fail to provide accurate information on subject body composition due to its major limitation in distinguishing between lean body mass (LBM) and fat body mass. Actually, LBM takes up approximately 75–90% of body weight in normal adults (6). Thus, it may be more reasonable to regard BMI as a rough proxy to assess LBM since BMI largely reflects total body weight rather than fat body weight.
Compared to BMI, fat-free mass index (FFMI), which is calculated by the total LBM divided by the square of height, offers a much better discriminatory power for LBM and acts as a superior surrogate for physical fitness (6,7). The clinical significance of FFMI has been explored in cardiac and colorectal surgery, showing a potent predictive value for several major complications (8,9). However, unlike BMI, the impact of FFMI on risk of post-lobectomy PAL has never been elucidated until recently.
Objectives
The primary purpose of our study was to estimate whether FFMI could be predictive of PAL complicating video-assisted thoracoscopic surgery (VATS) lobectomy for operable non-small-cell lung cancer (NSCLC). Our secondary goal was to explore the effects of FFMI on the length of stay (LOS) and time to air leak cessation after surgery.
Methods
Design and protocol
This single-center retrospective study was conducted on the prospectively-maintained database in our institution. We wrote it in compliance with the Strengthening the Reporting of Cohort Studies in Surgery Statement (Table S1) (10). The study protocol was approved by our Regional Ethics Committee (ID: 2016-255).

Full table
Patient selection
Settings
We retrospectively reviewed the clinical data of consecutive patients undergoing VATS lobectomy for operable NSCLCs at our unit between January 2015 and July 2017. All available data for patient characteristics were extracted from our medical records.
Eligibility criteria
The following eligibility criteria were utilized to determine the appropriateness of patients included:
- The target diseases were operable primary NSCLCs;
- Only standardized single-lobectomy with systematic mediastinal lymph node dissection (SMLND) operated by a completely VATS procedure would be included. Any additional surgical procedure, such as conversion to thoracotomy or extended resection, was not considered;
- Patients who had finished our standardized clinical pathways during the hospitalization were included (11);
- Patients who received neoadjuvant therapy were not considered, in order to avoid any confounding influence from a potential weight loss induced by neoadjuvant therapy, which might complicate the actual roles of baseline FFMI.
- Patients with loss of accurate medical records would not be considered.
Outcome data, measures and definitions
We recorded and defined the following characteristics and outcome data.
Preoperative parameters
Baseline information included age, gender, BMI, FFMI, body fat percentage (BF%), forced expiratory volume in one second (FEV1), FEV1 to forced volume capacity ratio (FEV1/FVC) and smoking history (11-17).
Body composition assessment criteria were as follows:
- Body height and weight of included patients were measured by our experienced nurses with standard methods;
- BMI (kg/m2) was calculated by weight (kg)/height (m)2;
- We determined to utilize the Clínica Universidad de Navarra-Body Adiposity Estimator (CUN-BAE) equation to calculate the BF% due to its good validity with remarkable consistency with actual BF% and great accessibility in a large population (7): BF%=−44.988+(0.503×age)+(10.689×gender)+(3.172×BMI)−(0.026×BMI2)+(0.181×BMI×gender)−(0.02×BMI×age)−(0.005×BMI2×gender)+(0.00021×BMI2×age)(1-male;0-female);
- We finally calculated the FFMI (kg/m2) as: FFMI=(1−BF%)×BMI (7).
Preoperative underlying comorbidities included respiratory comorbidity (comprising of chronic obstructive pulmonary disease, emphysema, lung bullae, tuberculosis, asthma, pneumonia, bronchiectasis, lung abscess and interstitial lung diseases), cardio-cerebrovascular comorbidity (comprising of hypertension, coronary heart diseases, peripheral arterial diseases, stroke, aortic aneurysm and chronic heart failure), diabetes mellitus, renal insufficiency, previous malignancy and steroid use (11-17).
Intraoperative parameters
Estimated intraoperative variables included the tumor location, presence of dense pleural adhesion (15), pulmonary fissure completeness (11,14), estimated intraoperative blood loss (EIBL) (16) and operation time.
Pathological parameters
The following four pathological variables were assessed: histological subtypes, tumor invasion (T-stage), lymph node metastasis (LNM) (N-stage) and pathological TNM-stage, all of which were in compliance with the Union for International Cancer Control Seventh Edition (11-17).
Outcomes of interest
The primary outcome of interest was postoperative PAL. A diagnosis of PAL was determined by an air leak lasting >5 PODs detected from the chest drainage system, which was judged in compliance with the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definition (18).
Our secondary outcomes were the LOS and time to air leak cessation. The LOS was measured from the operation day to the discharge day. The air leak duration was referred to the days that an air leak persists after surgery.
Grouping criteria
Taking account of significant ethnic differences between Chinese and Western populations, our BMI categorization was in compliance with the China’s National Health and Family Planning Commission definitions (underweight: <18.5 kg/m2; normal: 18.5 to <24 kg/m2; overweight: 24 to <28 kg/m2; obese: ≥28 kg/m2) (6).
Many healthy population cross-sectional surveys had been published to describe the body composition classifications, but unfortunately, neither international nor Chinese consensus was thus recommended on the reference values for FFMI (6-9,19). Therefore, we consulted a similar grouping criterion reported by Ekström et al. (19) in their body composition analysis. The gender-specific median values of FFMI in male and female patients were determined as the cutoffs to stratify our cohort of patients into the low-FFMI group and the high-FFMI group.
Surgical procedure and chest tube management
Our VATS lobectomy with SMLND was operated through a three-portal access, using a modified ‘hilum-first-fissure-last’ thoracoscopic technique known as “single-direction lobectomy” (11-17). Mechanical staplers were implemented in all patients to divide the incomplete inter-lobar fissures and close the bronchial stumps. Neither topical sealant nor pleural tenting was utilized in this period. At the end of the operation, we submerged the inflated lung parenchyma (25–30 cmH2O pressure) in warm sterile saline to examine whether an air leak was present. If air leaks were visually observed, then we would attempt to repair the parenchymal source of bubbles by applying sutures. Finally, one 20 Fr chest tube was placed within the hemithorax and attached to a conventional chest drainage system at −10–20 cmH2O suction, and then the wounds were stitched.
Chest tube was left on the suction until the morning of POD 1, and then was converted to water seal when minimal or no air leak was evident. Lung recruitment was shown by chest radiography. The presence of air leak was determined by visualizing the bubbles from the chest drainage system when patients were instructed to perform standardized repeated forced expiratory maneuvers (coughing/blowing) in an upright sitting position. The quantification of air leak was measured according to the Robert-David-Cerfolio Classification System (20). It was documented by our board-certified attending surgeons at least twice daily during the morning and evening rounds. An air leak that ceased until the morning of POD 1 was defined as ‘no air leak’, but an air leak persisting >5 days was termed as PAL (18). Once PAL was diagnosed, we placed the chest tube on the suction device and began to follow the management algorithm described by Okereke et al. (3). Chest tube removal was permitted when air leak cessation was detected from the chest drainage system and 24-hour pleural drainage <200 mL.
Statistical analysis
We employed the Pearson’s chi-squared test with Yates correction or Fisher’s exact-test, as appropriate, to compare the categorical variables (number with percentage), and the Mann-Whitney U-test to compare the continuous variables [mean ± standard deviation and median with interquartile range (IQR) (25th–75th quartile-interval)] (5,9,19). Effects of dichotomized FFMI on the air leak duration and LOS were estimated by a Kaplan-Meier analysis using the log-rank test.
A receiver operating characteristic (ROC) analysis was conducted to determine the discriminative power of continuous FFMI for the prediction of PAL. Area under curve (AUC) with its 95% confidence interval (CI) was then calculated.
Finally, gender-specific median FFMI and other clinicopathological variables with P<0.10 were included in a multivariable binary logistic-regression model, which utilized the Hosmer-Lemeshow test for goodness-of-fit and the C-statistic for discrimination, to identify the independent risk factors for PAL (5,21). In order to provide concise and informative factors for the prediction of PAL, the continuous variables were dichotomized in accordance with their clinically meaningful cutoffs that were generally accepted for risk stratification in routine clinical practice, including the geriatric state categorized by age >65 years, underweight state defined by BMI <18.5 kg/m2, impaired lung function categorized by FEV1% <80 and FEV1/FVC% <70, larger VATS blood loss categorized by EIBL >100 mL and prolonged operation time >150 min. Odds ratio (OR) with 95% CI was then obtained.
To eliminate potential confounding influence from an inextricable connection between age, gender, BMI and FFMI, we also included the dichotomized data of these baseline characteristics in multivariable logistic-regression model, even though they had no statistical significance in univariable analysis.
We used the IBM SPSS 22.0 software to accomplish above statistical analyses. The statistical significance was indicated by P value <0.05.
Results
Basic information and outcomes
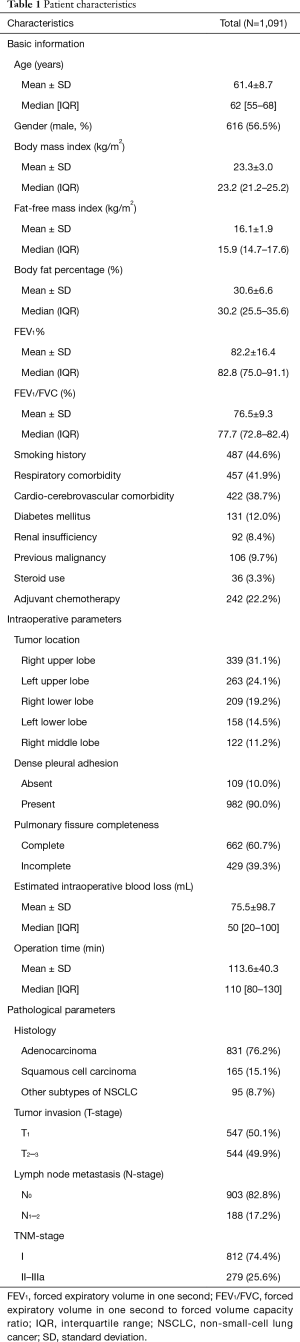
Patient characteristics
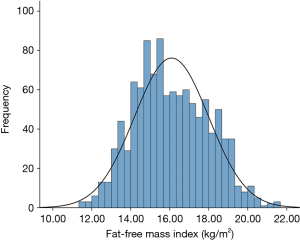
During the study period, there were 1,091 patients undergoing VATS lobectomy for operable primary NSCLCs included. Their patient characteristics are presented in Tables 1,2. Our cohort were comprised of 616 male (ratio =56.5%) and 475 female patients (ratio =43.5%), with a mean age of 61.4±8.7 years (median =62 years; IQR =55–68 years). The mean BMI, FFMI and BF% of the entire cohort was 23.3±3.0 kg/m2 (median =23.2 kg/m2; IQR =21.2–25.2 kg/m2), 16.1±1.9 kg/m2 (median =15.9 kg/m2; IQR =14.7–17.6 kg/m2) and 30.6±6.6 (median =30.2; IQR =25.5–35.6), respectively. A frequency distribution histogram suggested that our FFMI data could be approximately seen as the normal distribution (Figure 1).
Full table
Full table
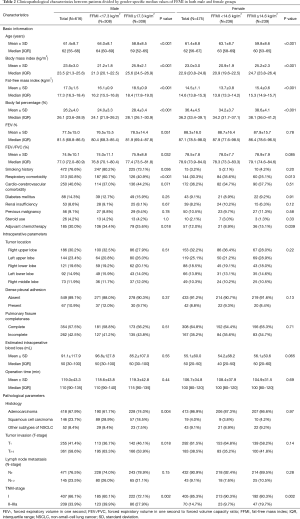
The mean FFMI for males and females was 17.3±1.5 kg/m2 (median =17.3 kg/m2; IQR =16.3–18.4 kg/m2) and 14.5±1.1 kg/m2 (median =14.6 kg/m2; IQR =13.8–15.3 kg/m2), respectively. The gender-specific median values of FFMI were further utilized to divide all included patients into the low-FFMI group (male FFMI <17.3 kg/m2, n=308; female FFMI <14.6 kg/m2, n=236) and the high-FFMI group (male FFMI ≥17.3 kg/m2, n=308; female FFMI ≥14.6 kg/m2, n=239). Patient characteristics between these groups are further summarized in Table 2.
Outcomes
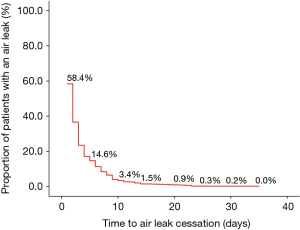
An air leak was detected in 58.4% of patients on POD 1 (n=637). Then, the proportion of air leak cases showed a steady decreasing tendency with the increasing LOS (Figure 2). Among these cases, 478 of them showed a completely ceased air leak within 5 days, but the remaining 159 patients developed an air leak persisting>5 days. Thus, the overall PAL incidence in our series was 14.6%. There was no in-hospital death. All included patients were discharged without remaining chest tube drainage. In addition, the mean LOS and air leak duration in our series was 6.5±4.3 days (median =6 days; IQR =4–8 days) and 2.8±3.6 days (median =2 days; IQR =0–3 days) respectively.
Comparisons between patients with and without PAL
Male group
Among 616 male patients, a PAL occurred in 117 individuals, with an incidence of 19.0%. PAL cases had significantly higher ratios of respiratory comorbidity (P<0.001), cardio-cerebrovascular comorbidity (P=0.028), incomplete pulmonary fissure (P<0.001), dense pleural adhesion (P=0.016), T2–3-stage tumor invasion (P=0.030), LNM (P=0.006) and II–IIIa-stage cancer (P=0.014) than those of non-PAL cases (Table 3).
Full table
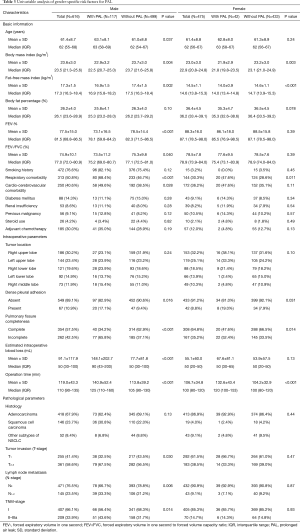
PAL cases also had significantly higher means of age (P=0.037), EIBL (P<0.001) and operation time (P<0.001) but lower means of FEV1% (P<0.001) and FEV1/FVC% (P=0.040) than those of non-PAL cases (Table 3). Both mean FFMI (16.9±1.5 vs. 17.4±1.5 kg/m2; P=0.002) and BMI (22.9±3.2 vs. 23.7±3.0 kg/m2; P=0.004) in PAL cases were significantly lower than those in non-PAL cases (Figure 3A,B,C). Moreover, the incidence of PAL in patients with FFMI <17.3 kg/m2 was significantly higher than that in patients with FFMI ≥17.3 kg/m2 (23.7% vs. 14.3%; P=0.003).
Female group
There were 42 of 475 female patients experienced a PAL, with an incidence of 8.8%. Compared to non-PAL cases, PAL cases had a significantly higher mean operation time (P<0.001) and significantly higher ratios of respiratory comorbidity (P=0.011), dense pleural adhesion (P=0.031) and incomplete pulmonary fissure (P=0.014) (Table 3). Moreover, both mean FFMI (14.0±0.9 vs. 14.6±1.1 kg/m2; P<0.001) and BMI (21.9±2.9 vs. 23.2±3.0 kg/m2; P=0.003) in PAL cases were significantly lower than those in non-PAL cases (Figure 3A,B,C). The incidence of PAL in patients with FFMI <14.6 kg/m2 was significantly higher than that in patients with FFMI ≥14.6 kg/m2 (12.7% vs. 5.0%; P=0.003).
ROC analysis of continuous FFMI for predicting postoperative PAL
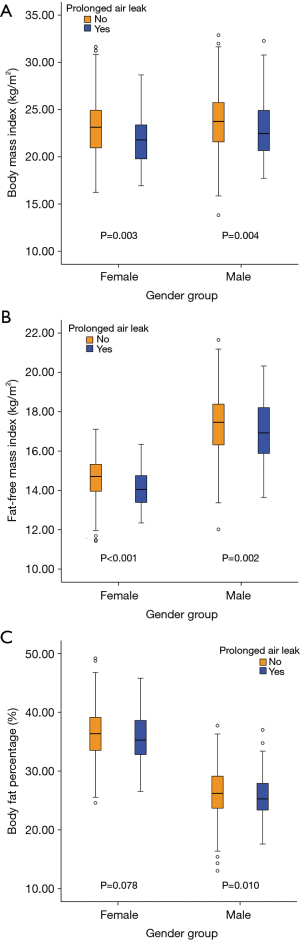
The ROC analysis of continuous FFMI showed an AUC of 0.59 (95% CI: 0.54–0.65; P=0.002) in male group and an AUC of 0.67 (95% CI: 0.59–0.75; P<0.001) in female group for the prediction of PAL (Figure 4A,B). The male median FFMI of 17.3 kg/m2 showed 53.3% sensitivity and 62.4% specificity, while the female median FFMI of 14.6 kg/m2 showed equal sensitivity at 53.3% but higher specificity at 71.4% with regard to risk of PAL.
Multivariable analysis of risk factors for PAL
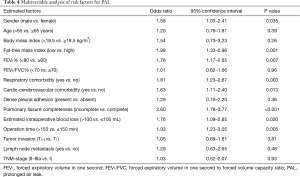
A multivariable logistic-regression model was formulated on the clinicopathological parameters with P<0.10 in both male and female patients, as shown in Table 4. The multivariable logistic-regression model with Hosmer-Lemeshow P=0.59 and C-statistic =0.77 (95% CI: 0.73–0.81; P<0.001) demonstrated that the male gender (OR =1.58; 95% CI: 1.03–2.41; P=0.035), lower dichotomized FFMI (OR =1.98; 95% CI: 1.33–2.96; P=0.001), FEV1% <80 (OR =1.76; 95% CI: 1.17–2.65; P=0.007), respiratory comorbidity (OR =1.81; 95% CI: 1.23–2.67; P=0.003), cardio-cerebrovascular comorbidity (OR =1.63; 95% CI: 1.11–2.40; P=0.013), poorly-developed pulmonary fissure (OR =2.60; 95% CI: 1.78–3.77; P<0.001), operation time >150 min (OR =1.93; 95% CI: 1.23–3.05; P=0.005) and EIBL >100 mL (OR =1.76; 95% CI: 1.09–2.85; P=0.020) could independently predict the occurrence of PAL. No significant association was found between BMI <18.5 kg/m2 and postoperative PAL (OR =1.54; 95% CI: 0.73–3.23; P=0.26).
Full table
Effects of dichotomized FFMI on air leak duration and LOS
Time to air leak cessation
The Kaplan-Meier curve revealing time to air leak cessation between low-FFMI group and high-FFMI group are presented in Figure 5A. The air leak duration in low-FFMI group patients (mean =3.2 days; 95% CI: 2.9–3.6 days) was significantly longer than that in high-FFMI group patients (mean =2.3 days; 95% CI: 2.1–2.5 days) (Log-rank P<0.001).
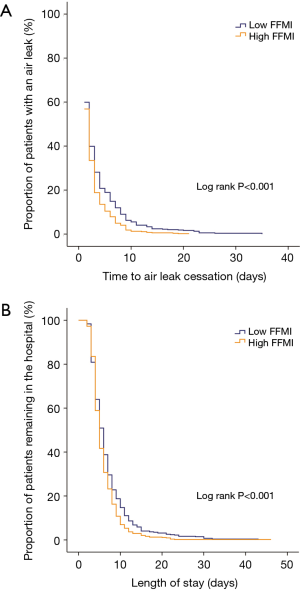
LOS
Figure 5B shows the LOS between low-FFMI group and high-FFMI group. The Kaplan-Meier analysis indicated that low-FFMI group patients (mean =6.9 days; 95% CI: 6.5–7.4 days) had the significantly prolonged LOS compared with that of high-FFMI group patients (mean =6.0 days; 95% CI: 5.7–6.3 days) (Log-rank P<0.001).
Discussion
Key results and interpretations
Prior prospective studies have demonstrated a significant influence of FFMI on major outcomes complicating cardiac and colorectal surgery (8,9). In these surgical specialties, low FFMI was regarded as one of the most powerful factors for distinguishing undernourished surgical patients and enhanced recovery after surgery (8,9). To the best of our knowledge, the present study was the first to demonstrate the predictive value of FFMI for risk of PAL and time to air leak cessation following VATS lobectomy for NSCLC. We selected a series of widely accepted formulas comprising of BMI, age and gender, rather than a classical dual-energy X-ray absorptiometry (DEXA), to extrapolate the FFMI data of a large cohort consisting of >1,000 cases, as these formulas have been validated with great consistency with the actual FFMI (7). The DEXA scan might not be easily implemented in a large population because of a high cost and a little complex process, although it was considered the gold standard for body composition measurement (6,7,9).
The main finding of our study was that both male and female patients with lower dichotomized FFMI were considered to suffer from a significantly higher risk of PAL complicating VATS lobectomy for NSCLC. Furthermore, both air leak duration and LOS were significantly prolonged in the patients with lower dichotomized FFMI. That might be attributed to a higher incidence of PAL among these patients. Finally, an effective multivariable logistic-regression model demonstrated that lower dichotomized FFMI could independently predict the occurrence of PAL in both male and female patients. Low BMI (underweight state) was also identified to predispose to PAL formation but did not reach statistical significance. These findings were consistent with the previous results in other surgical specialties, revealing a superior clinical significance of FFMI compared with BMI (8,9). Although potential mechanisms underlying the association between low FFMI and risk of PAL remain unclear, we hypothesize that the following three explanations may be considered when trying to explain this phenomenon.
First, the LBM, particularly in the form of muscular tissues, contains abundant proteins that maintain every physiological process within somatic cells, including the synthesis of essential enzymes for metabolic responses and active antibodies for immunological reactions, cell signaling, and cell regeneration. As an excellent indicator for LBM, low FFMI may represent an insufficient status of organism nutritional and physiological reserve, implying a large decline of metabolically active somatic cells (8,22). The excessive protein catabolism induced by surgery can further impair the global immunological function and physiological homeostasis. Therefore, patients with lower FFMI are less likely to have an adequate response to operative stress because of their compromised ability to withstand an acute injury, resulting in a dramatically increased risk of adverse events (22). In addition, any operative morbidity in such patients cannot be easily managed and typically requires a prolonged convalescence period after surgery, otherwise, a threshold at which the organism ceases to function will eventually be reached (9,22).
Second, we speculated that a positive correlation between FFMI and major cardiopulmonary function indices might affect the development of PAL. Accumulative evidence demonstrates that each 1 kg/m2 increase in FFMI is linearly associated with an increasing FEV1% (23,24). In other words, a lower FFMI can reflect impaired lung function based on a decline of FEV1%, which represents one of the leading risk factors for PAL (2). This phenomenon suggests that a loss of LBM, particularly of the respiratory muscles within the thorax and upper abdomen, diminishes the capacity of breathing exercises, leading to a downtrend of FEV1% and tidal volume. Recently, a small cross-sectional study reported that each 1 kg/m2 decrease in FFMI may be responsible for a decreasing maximal oxygen consumption, which is one of the best measurements reflecting the cardiopulmonary capacity, as the fewer muscular pumps participating in physical activities contribute to a lower venous return to the heart (25). That may be another one predisposing factor for PAL.
Finally, as a powerful risk factor for PAL, COPD is characterized by a range of pathophysiological changes, and one of its main consequences is the progressive wasting of LBM, resulting in the presence of bio-energetic abnormality (26). A decline of physical activities and long-term glucocorticoid administration in COPD patients further contributes to a loss of muscular mass, which is typically accompanied by an abundance of adiposity. Thus, compared to normal patients, most COPD patients have a lower FFMI and present with a steady downtrend of LBM with the increasing severity of disease, showing a significantly higher probability to experience a PAL (26,27).
Generalizability
Our findings suggest that FFMI may be a more appropriate alternative compared to BMI for the formulation of a novel risk scoring system to help thoracic surgeons stratify the patients at high surgical risk. In addition, we suggest that the present results may help to select the candidates in a teaching program of VATS techniques or in an early learning curve for young surgeons to avoid adverse events and train them more effectively.
Limitations
The following major study limitations must be acknowledged.
First, the present study was subject to inherent limitations of any single-center retrospective analysis. Potential selection bias might complicate our findings, although we included >1,000 patients in accordance with fairly strict eligibility criteria and performed a more appropriate gender-specific risk analysis by multivariable logistic-regression model on the cohort, in order to eliminate potential bias risks from confounding factors, including the application of neoadjuvant therapy, gender heterogeneity, FFMI extrapolation and additional surgical procedures. For example, long-term smoking and consumptive respiratory diseases, such as the COPD, chronic bronchitis and tuberculosis history, could lead to a loss of LBM and impaired lung function before surgery, resulting in a significantly increased probability of PAL. In addition, dissection of the lung parenchyma within the poorly-developed fissure and dense pleural adhesion could significantly prolong the operation time and easily produce a PAL requiring chest suction drainage or even surgical intervention. Given such concerns, we recommend that more prospective validating studies with much better control of potential confounders from patient characteristics are needed to demonstrate the significance of FFMI in lung cancer surgery.
Second, neither FFMI nor BF% in our large series was directly measured by the ‘gold-standard’ DEXA scan. Nevertheless, we estimated both FFMI and BF% according to the Lavie formula and CUN-BAE equation (7), which have been validated in many large populations and readily employed to assess body composition without any specific equipment or additional cost. However, a further comparison with other calculating formulas of FFMI in a large population is warranted to verify the effectiveness of our findings in the future.
Third, the AUCs of continuous FFMI in both male and female groups were relatively low but with P-values near 0.001 for the prediction of PAL. These results might not provide a strong discriminatory power for the continuous FFMI. Furthermore, the gender-specific median values of FFMI showed fairly low sensitivity and specificity, which might attenuate the practical purpose of our findings in routine clinical practice.
Fourth, some of pulmonary diffusion function indexes, such as the carbon monoxide diffusing capacity, had unfortunately missed from our database maintained during the study period. So these parameters were not evaluated in all included patients.
Finally, the PAL incidence could also depend on the surgeons’ expertise and conditions. However, it might be difficult to appropriately perform a quantitative analysis on these artificial factors. Besides, we paid much less attention to the treatment of PAL due to the restriction of primary study objectives.
Conclusions
In conclusion, the present study demonstrates that FFMI can serve as an excellent categorical predictor for PAL complicating VATS lobectomy for NSCLC. Moreover, FFMI was found to be more promising than BMI in terms of the prediction of PAL. It may be extremely helpful to incorporate a FFMI cutoff into perioperative risk assessment models. Owing to several inherent limitations of the retrospective design, more large-scale prospective validating analyses are highly recommended to confirm and modify our findings in the future.
Acknowledgments
We give special thanks to Mrs. Hong Xie, from the Institution of Medical English, West China Medical Center, Sichuan University, Chengdu, China, for her help with the English language editing of this article.
Funding: This study was supported by the Foundation of Science and Technology support plan Department of Sichuan Province (2015SZ0158).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by our Regional Ethics Committee (ID: 2016-255).
References
- Li SJ, Zhou K, Li YJ, et al. Efficacy of the fissureless technique on decreasing the incidence of prolonged air leak after pulmonary lobectomy: A systematic review and meta-analysis. Int J Surg 2017;42:1-10. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Okereke I, Murthy SC, Alster JM, et al. Characterization and importance of air leak after lobectomy. Ann Thorac Surg 2005;79:1167-73. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Attaar A, Winger DG, Luketich JD, et al. A clinical prediction model for prolonged air leak after pulmonary resection. J Thorac Cardiovasc Surg 2017;153:690-699.e2. [Crossref] [PubMed]
- Lu Y, Shu H, Zheng Y, et al. Comparison of fat-free mass index and fat mass index in Chinese adults. Eur J Clin Nutr 2012;66:1004-7. [Crossref] [PubMed]
- Huang BT, Peng Y, Liu W, et al. Lean mass index, body fat and survival in Chinese patients with coronary artery disease. QJM 2015;108:641-7. [Crossref] [PubMed]
- van Venrooij LM, De Vos R, Zijlstra E, et al. The impact of low preoperative fat-free body mass on infections and length of stay after cardiac surgery: a prospective cohort study. J Thorac Cardiovasc Surg 2011;142:1263-9. [Crossref] [PubMed]
- Tsaousi G, Kokkota S, Papakostas P, et al. Body composition analysis for discrimination of prolonged hospital stay in colorectal cancer surgery patients. Eur J Cancer Care (Engl) 2017. [Crossref] [PubMed]
- Agha RA, Borrelli MR, Vella-Baldacchino M, et al. The STROCSS statement: Strengthening the Reporting of Cohort Studies in Surgery. Int J Surg 2017;46:198-202. [Crossref] [PubMed]
- Li S, Zhou K, Wang M, et al. Degree of pulmonary fissure completeness can predict postoperative cardiopulmonary complications and length of hospital stay in patients undergoing video-assisted thoracoscopic lobectomy for early-stage lung cancer. Interact Cardiovasc Thorac Surg 2018;26:25-33. [Crossref] [PubMed]
- Li S, Zhou K, Du H, et al. Body surface area is a novel predictor for surgical complications following video-assisted thoracoscopic surgery for lung adenocarcinoma: a retrospective cohort study. BMC Surg 2017;17:69. [Crossref] [PubMed]
- Li SJ, Zhou K, Shen C, et al. Body surface area: a novel predictor for conversion to thoracotomy in patients undergoing video-assisted thoracoscopic lung cancer lobectomy. J Thorac Dis 2017;9:2383-96. [Crossref] [PubMed]
- Li S, Wang Z, Zhou K, et al. Effects of degree of pulmonary fissure completeness on major in-hospital outcomes after video-assisted thoracoscopic lung cancer lobectomy: a retrospective-cohort study. Ther Clin Risk Manag 2018;14:461-74. [Crossref] [PubMed]
- Li SJ, Zhou K, Wu YM, et al. Presence of pleural adhesions can predict conversion to thoracotomy and postoperative surgical complications in patients undergoing video-assisted thoracoscopic lung cancer lobectomy. J Thorac Dis 2018;10:416-31. [Crossref] [PubMed]
- Li S, Zhou K, Lai Y, et al. Estimated intraoperative blood loss correlates with postoperative cardiopulmonary complications and length of stay in patients undergoing video-assisted thoracoscopic lung cancer lobectomy: a retrospective cohort study. BMC Surg 2018;18:29. [Crossref] [PubMed]
- Li S, Wang Y, Zhou K, et al. Body surface area as a novel risk factor for chylothorax complicating video-assisted thoracoscopic surgery lobectomy for non-small cell lung cancer. Thorac Cancer 2018;9:1741-53. [Crossref] [PubMed]
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Ekström E, Ansari D, Williamsson C, et al. Impact of body constitution on complications following pancreaticoduodenectomy: A retrospective cohort study. Int J Surg 2017;48:116-21. [Crossref] [PubMed]
- Cerfolio RJ. Chest tube management after pulmonary resection. Chest Surg Clin N Am 2002;12:507-27. [Crossref] [PubMed]
- Domínguez-Almendros S, Benítez-Parejo N, Gonzalez-Ramirez AR. Logistic regression models. Allergol Immunopathol (Madr) 2011;39:295-305. [Crossref] [PubMed]
- Tsai S. Importance of lean body mass in the oncologic patient. Nutr Clin Pract 2012;27:593-8. [Crossref] [PubMed]
- Alvarez JA, Ziegler TR, Millson EC, et al. Body composition and lung function in cystic fibrosis and their association with adiposity and normal-weight obesity. Nutrition 2016;32:447-52. [Crossref] [PubMed]
- Cotes JE, Chinn DJ, Reed JW. Body mass, fat percentage, and fat free mass as reference variables for lung function: effects on terms for age and sex. Thorax 2001;56:839-44. [Crossref] [PubMed]
- Mondal H, Mishra SP. Effect of BMI, Body Fat Percentage and Fat Free Mass on Maximal Oxygen Consumption in Healthy Young Adults. J Clin Diagn Res 2017;11:CC17-20. [PubMed]
- Ischaki E, Papatheodorou G, Gaki E, et al. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest 2007;132:164-9. [Crossref] [PubMed]
- Kim SB, Kang YA, Jung JY, et al. Body mass index and fat free mass index in obstructive lung disease in Korea. Int J Tuberc Lung Dis 2014;18:102-8. [Crossref] [PubMed]