Stand-alone surgical ablation for atrial fibrillation: a novel bilateral double-port approach
Introduction
Atrial fibrillation (AF) is one of the most common arrhythmias. In 2010, it was estimated that there were approximately 20 million male AF patients and 12 million female AF patients worldwide (1). AF increases the risk of stroke and death, lowers left heart function and quality of life, and increases both personal and societal healthcare costs (2-4). As a consequence, the importance of AF treatment has been increasingly recognized by clinicians and health authorities.
The treatments of AF include anticoagulant therapy, rate control therapy, and rhythm control therapy. Anticoagulation is the foundation therapy to lower risk of stroke or thromboembolism. Rhythm control therapy for the management of AF is used is to improve quality of life, lower risk of heart failure progression, and decrease heart failure hospitalizations. Rhythm control can be obtained through electrical or chemical cardioversion, antiarrhythmic drug therapy, or nonpharmacologic approaches. Surgical and catheter-based ablations, if successful, can lead to long-term rhythm benefit without chronic exposure to drug therapies. Since the landmark Cox-Maze procedure (CMP) was first described in 1987 (5), there has been considerable progress in the surgical treatment of AF. The classic CMP III has definite effectiveness and remains the gold-standard surgical treatment for AF. However, it is not widely applied due to its invasiveness and technical complexity. Although the modified CMP IV is technically easier and less invasive, it still needs to be performed under extracorporeal circulation and sternotomy, which was not accepted by patients of lone AF and cardiologists soundly. To further reduce surgical trauma and increase patient acceptance, Dr. Wolf, La Meir and Mei each introduced a different thoracoscopic mini-maze procedure (MMP) independently (6-11). Based on these previous experiences and innovations, we have developed a novel bilateral double-port totally endoscopic approach for MMP and achieved good surgical results.
Methods
Patient population
This retrospective study was approved by the Institutional Review Board (IRB) of Guangdong Provincial People’s Hospital. A total of 54 patients underwent a novel bilateral double-port totally endoscopic ablation (BDTEA) from January 2016 to June 2017. After detailed history-taking, physical examination, a CHA2DS2-VASc scoring was calculated. Transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE) were performed in all patients to rule out left atrial thrombus. A chest computed tomography (CT) angiogram with 3D reconstruction was also performed in all patients to assess left atrial anatomy. All patients signed the informed consents before surgery. Surgical indications include: (I) lone AF patients with subjective symptoms who have failed antiarrhythmic drug therapy; (II) patients unresponsive to medical cardioversion or catheter ablation; or, (III) AF patients with a history of stroke or cerebral thrombosis, along with left ventricular ejection fraction (LVEF) >30%. Relative contraindications include: (I) history of left or right thoracic surgery; (II) history of pleural adhesions; (III) history of heart and/or lung surgery; (IV) atrial thrombus; and/or (V) left atrial diameter >55 mm.
Surgical procedures
Surgical process
The patient was placed in a supine position with inflatable airbags of 1,000 mL volume under each scapula to raise right or left lateral chest. General anesthesia was performed with double-lumen endotracheal intubation. External defibrillation pads were placed near the apex and on the right shoulder. Endocardial pacing electrodes were routinely placed via the internal jugular vein for provisory pacemaker.
Right approach
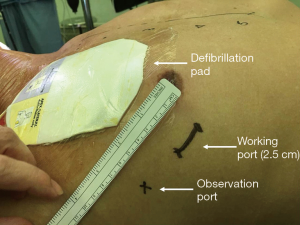
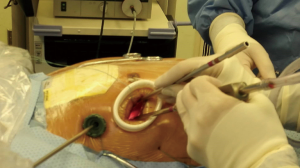
The airbag under the right scapula was inflated to raise the right chest, and the left lung was ventilated unilaterally. A 2.5-cm incision was made in the 4th intercostal space at right anterior axillary line and extended with tissue retractor as working port (for female patients, a curve incision was made 1 cm above the breast fold, and approach to the thoracic cavity was through the fourth intercostal space as well via a subcutaneous tunnel), and a 1.0-cm incision was made in the 4th intercostal space at right middle axillary line as the thoracoscopic observation port (Figures 1,2). The pericardium was opened 2 cm above the phrenic nerve and suspended to expose the heart. The fat pad in the interatrial groove was divided with electric cautery. After blunt dissection of transverse and oblique sinuses, Wolf Dissector (AtriCure. Inc., Ohio, USA) was utilized to snare the right pulmonary veins via the right inferior pulmonary vein towards left atrial dome. Then AtriCure bipolar isolator (AtriCure. Inc., Ohio, USA) was introduced via the snare catheter. The tip of the isolator should point to the left appendage as close as possible so that the ablation line could be connected with left side ablation to reach continuous transmural lesion of roof. Each line was ablated for 6 times. Then AtriCure bipolar ablation pen (AtriCure. Inc., Ohio, USA) was used to complete the ablation of roof line and floor line. A drainage catheter was inserted via thoracoscopic port, and the incisions were closed layer by layer.
Left approach
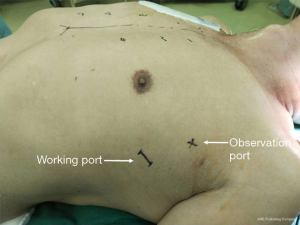
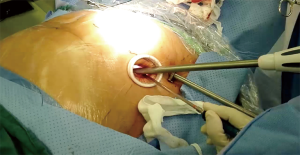
After right airbag deflation, the left airbag was inflated to elevate the left chest and the right lung was ventilated unilaterally. A 2.5-cm incision was made in the 4th intercostal space at the left middle axillary line and extended with tissue retractor as working port, and a 1.0-cm incision was made in the 3rd intercostal space at left posterior axillary line as observation port (Figures 3,4). The pericardium was opened 2 cm beneath the phrenic nerve and suspended to expose the heart. The left atrial appendage (LAA) was resected first with a stapler (Ethicon EndoSurgery, Ohio, USA), which allows better exposure of the atrial roof and ligament of Marshall. After left pulmonary veins been snared by Wolf Dissector, The AtriCure bipolar isolator was introduced via the catheter with the tip advanced medially to reach the right side of the left atrium (previous ablation line) as much as possible. This was followed by ablation of the left pulmonary veins with six applications of energy delivery. Ablation of the left pulmonary vein- LAA line, and the left part of the floor line was repeated with the AtriCure bipolar ablation pen to form a complete “Box Lesion” of the posterior wall of left atrium. The incisions were then closed following the same procedure described for the right side. External synchronous cardioversion was applied if AF persisted after the completion of all steps.
Post-operative management
Amiodarone 400 mg/day was administered on the first postoperative day and gradually decreased to 200 mg/day (typically at discharge). Warfarin was also administered for anticoagulation routinely. A review examination was scheduled three months after discharge. Amiodarone was stopped then in all patients whether they were in sinus rhythm or not, and the need for long-term anticoagulation was determined by the CHA2DS2-VASc score. Catheter mapping and ablation was recommended for recurrent atrial tachyarrhythmias.
Follow-up
Patients were followed up by outpatient visits and telephone interviews 3, 6, and 12 months after surgery and then annually afterwards. The symptoms, TTE and 7-day Holter findings were recorded. The first three months after surgery were considered as the “blanking period”. After a 3-month blank period, a relapse was confirmed if 7-day Holter revealed an atrial arrhythmia (AF, atrial flutter, or atrial tachycardia) persisted for more than 30 seconds (1).
Statistical analysis
The count data were presented as percentages, and the measurement data as mean ± standard deviations (
Results
Demographics
These 54 patients had an average age of 55.5±9.0 years and 15 were female (27.8%). Seventeen patients (31.5%) had a history of smoking. Additional co-morbidities included hypertension (n=26, 48.1%), diabetes (n=6, 11.1%), coronary heart disease (n=3, 5.6%), and chronic obstructive pulmonary disease (n=3, 5.6%). Previous stroke and catheter ablation were noted in 11 (20.4%) and 7 (13.0%) respectively. The average CHA2DS2-VASc score was 1.5±1.1. The duration of AF was 49.4±56.6 months. The type of AF was persistent, longstanding persistent and paroxysmal in 12 (22.2%), 40 (74.1%), and 2 (3.7%) respectively. The left atrial diameter (LAD) was 42.6±5.0 mm (Table 1).
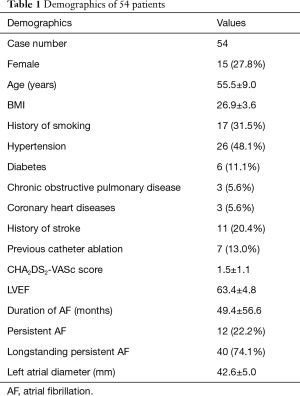
Full table
Hospitalization data
During the procedure, 1 patient (1.9%) was converted to sternotomy due to intraoperative hemorrhage and CMP IV was completed. Two patients underwent a one-stop hybrid procedure. In another two cases, only the right-sided ablation was performed due to left pleural adhesion, and they underwent subsequent percutaneous catheter ablation (CA) combined with LAA occlusion at day 6 and day 11 after surgery during the same hospitalization. The postoperative ventilation time was 8.6±8.6 hours, the ICU stay was 47.7±50.9 hours, and the postoperative hospital stay was 7.1±3.6 days. There was no in-hospital mortality. One patient required a repeat thoracoscopic exploration due to postoperative bleeding. Three patients developed mild lung infection and one suffered from bilateral diaphragm paralysis. No other complication was observed such as low cardiac output syndrome, stroke, permanent pacemaker implantation, or poor wound healing. Sinus rhythm was achieved before discharge in 26 patients (48.1%) (Table 2).
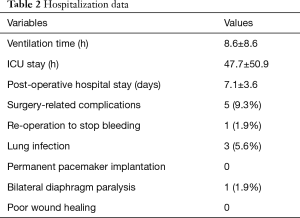
Full table
Follow-up data
All patients were seen in follow up with the mean length of follow-up of 14 months (range, 3–30 months). Although the one patient that converted to median sternotomy remained in sinus rhythm, the outcome was still counted as technique failure. Twenty-eight patients in sinus rhythm or not, underwent left atrium angiography to conform LAA closure (stump less than 1 cm in all patients), and catheter mapping and ablation three months after the operation to conform the lesion set made by this procedure. Sinus rhythm was achieved in 35 (64.8%) three months after surgery (before CA) and maintained in 47 (87.0%, after subsequent mapping & CA, P=0.005) without any anti-arrhythmia drug and all patients survived without stroke, hemorrhage or pulmonary vein stenosis in recent follow-up visits. The patient with symptomatic bilateral diaphragm paralysis improved gradually one month after discharge.
Discussion
CMP was first performed by James L. Cox in 1991 (5). It interrupts potential intra-atrial macroreentrant circuits and isolates triggers that cause AF through extensive “cut-and-sew” lesions in both the left and right atria. The procedure has favorable long-term efficacy at maintaining sinus rhythm, but with disadvantages that include a complex and prolonged operation, extensive surgical trauma, and alterations of atrial mechanics that can impact the chamber compliance and function. CMP III has gradually been replaced by CMP IV where “cut-and-sew” is replaced by radiofrequency, cryo or other energy for ablation to simplify the operation. Both CMP III and CMP IV need to be performed under extracorporeal circulation and sternotomy, mostly as a concomitant procedure in open-heart surgery.
In 2003, Wolf pioneered the clinical application of thoracoscopic assisted bilateral approach for surgical ablation, known as Wolf Mini-Maze Procedure (WMMP) (8,9), and achieved a sinus rhythm conversion rate close to that of the traditional CMP IV. Compared with WMMP, our procedure has the following advantages: first, patients that receive a WMMP need to be positioned and draped differently for the right and left steps of ablation. In contrast, during the procedure described here, inflatable airbags are placed under both shoulders. Through inflation and deflation of the airbags and by rotating the surgical table, the position of patients can be easily adjusted without compromising the contralateral surgical site. As a consequence, no re-disinfection or re-draping is needed, which helps to save time and effort and reduce the risk of surgical site infection. Next, only 2 small ports on each side of the chest are needed, resulting in better cosmetic results. Third, no iatrogenic pneumothorax is required. And most importantly, we can easily adjust the tips of clamp to reach as high as the roof of LA by each side (for continuous transmural lesion of roof) and make floor line by bipolar pen via the working port, to upgrade the simple pulmonary vein isolation (PVI) to a “box lesion” of the posterior wall of LA, which theoretically has a higher rate of success by isolating additional AF drivers.
Dr. La Meir introduced a bilateral three-port totally endoscopic approach procedure (7,12) which requires enough thoracoscopy experience and longer learning curve, and the roof line was difficult to ablate in this way. The left posterior lateral thoracoscopic MMP, invented by Dr. Mei in China, allowed for completion of the “box lesion” set for posterior wall isolation and a left atrial appendectomy with good effectiveness (10,11). However, this approach remains a highly challenging surgery. We had adopted it in our center; however, the proportion (22.2%) of conversion to sternotomy due to intraoperative hemorrhage was high. Therefore, we sought alternative approaches to maintain efficacy while improving operative feasibility and safety. Of the 54 patients in which our new procedure was attempted, only once did the surgery require conversion to sternotomy (1.9%), which happened in the early stage of our practice. In this patient, bleeding from left atrial dome developed while dissecting the transverse sinus. This bleeding may have been controlled after enlarging the main operation port. However, due to the lack of experience with this new technique and based on safety considerations, we decided to convert the surgery to sternotomy. The perioperative complication rate was 9.3%, primarily pulmonary infections. One patient did suffer bilateral paralysis of the diaphragm, which may have been due to thermal damage of phrenic nerves caused by electric cautery or the AtriCure bipolar isolator. There was no perioperative death, demonstrating that the procedure has good safety. In addition, the procedure has a rapid learning curve and we anticipate that it can be easily mastered by surgeons.
All of the above MMP could not make ablation on isthmus and coronary sinus where triggers or macroreentrant circuits often located. However, if the procedure is combined with CA, endocardial ablation can be delivered to complete any gaps in the surgical lines or target additional AF drivers. With these additional ablations, a full maze can be approximated, and a similar effectiveness to those of classical CMP may be achieved. In this cohort, 96% of patients suffered from non-paroxysmal AF which often needs multiple CA interventions without surgical cooperation. We achieved a favorable 64.8% rate of AF conversion at 3 months after the procedure, similar to the one-year conversion rate achieved by Boersma et al. in the FAST (atrial Fibrillation catheter Ablation versus Surgical ablation Treatment) study (13). Also, we noted a significant augmentation in success after supplemental CA with a conversion rate that reached 87.0%. In fact, several patients who failed surgical ablation didn’t receive supplementary CA for various reasons. So the rate of freedom from AF observed in this trial may be even higher if all the patients underwent a scheduled two-stage hybrid procedure.
Conclusions
In summary, this article reports the preliminary results of a novel MMP for the treatment of non-paroxysmal AF, with a small number of cases and short follow-up that demonstrate early feasibility and efficacy. This advocated procedure alone has many advantages including favorable safety, reproducibility, a rapid learning curve, and an encouraging effectiveness. And when combined with CA, as sequential hybrid procedure, will be the best solution for long-standing persistent AF.
Acknowledgments
Funding: This research was supported by the Grant of Guangdong Provincial People’s Hospital (2017zh06); the National Nature Science Foundation (81370295); the Science and Technology Planning Project of Guangdong Province, China (201508020261, 2017A070701013, 2017B090904034, 2017B030314109); and the Science and Technology Planning Project of Guangzhou (2014B070705005).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This retrospective study was approved by the Institutional Review Board (IRB) of Guangdong Provincial People’s Hospital [approval ID: No. GDREC2017290H(R1)]. Trial registration: ClinicalTrials.gov, NCT03708471. Registered on October 17, 2018. All patients signed the informed consents before surgery.
References
- Kirchhof P, Benussi S, Kotecha D, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with Eacts. Eur Heart J 2016;37:2893-962. [Crossref] [PubMed]
- Andersson T, Magnuson A, Bryngelsson I, et al. All-cause mortality in 272 186 patients hospitalized with incident atrial fibrillation 1995–2008: a Swedish nationwide long-term case–control study. Eur Heart J 2013;34:1061-7. [Crossref] [PubMed]
- Benjamin EJ, Wolf PA, D'Agostino RB, et al. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation 1998;98:946-52. [Crossref] [PubMed]
- Stewart S, Hart CL, Hole DJ, et al. A population-based study of the long-term risks associated with atrial fibrillation: 20-year follow-up of the Renfrew/Paisley study. Am J Med 2002;113:359-64. [Crossref] [PubMed]
- Cox JL, Schuessler RB, D'Agostino HJ, et al. The surgical treatment of atrial fibrillation. III. Development of a definitive surgical procedure. J Thorac Cardiovasc Surg 1991;101:569-83. [PubMed]
- La Meir M, De Roy L, Blommaert D, et al. Treatment of lone atrial fibrillation with a right thoracoscopic approach. Ann Thorac Surg 2007;83:2244-5. [Crossref] [PubMed]
- Pison L, La Meir M, van Opstal J, et al. Hybrid thoracoscopic surgical and transvenous catheter ablation of atrial fibrillation. J Am Coll Cardiol 2012;60:54-61. [Crossref] [PubMed]
- Wolf RK. Treatment of lone atrial fibrillation: minimally invasive pulmonary vein isolation, partial cardiac denervation and excision of the left atrial appendage. Ann Cardiothorac Surg 2014;3:98-104. [PubMed]
- Wolf RK, Schneeberger EW, Osterday R, et al. Video-assisted bilateral pulmonary vein isolation and left atrial appendage exclusion for atrial fibrillation. J Thorac Cardiovasc Surg 2005;130:797-802. [Crossref] [PubMed]
- Ma N, Ding F, Chen W, et al. Minimally invasive surgery via left thoracic approach for lone atrial fibrillation. Chinese Journal of Thoracic and Cardiovascular Surgery 2013;29:692-3.
- Ma N, Jiang Z, Yin H, et al. Mei mini maze procedure: experience of consecutive 353 patients and mean 2-year follow-up in single center. Chinese Journal of Thoracic and Cardiovascular Surgery 2015;31:670-3.
- de Asmundis C, Chierchia GB, Mugnai G, et al. Midterm clinical outcomes of concomitant thoracoscopic epicardial and transcatheter endocardial ablation for persistent and long-standing persistent atrial fibrillation: a single-centre experience. Europace 2017;19:58-65. [PubMed]
- Boersma LVA, Castella M, van Boven W, et al. Atrial Fibrillation Catheter Ablation Versus Surgical Ablation Treatment (FAST): A 2-Center Randomized Clinical Trial. Circulation 2012;125:23-30. [Crossref] [PubMed]