
Clinical outcomes following an initial experience with a novel powered vascular stapler in video-assisted thoracoscopic lobectomies: results of a Chinese multi-center study
Introduction
The video-assisted thoracoscopic surgery (VATS) lobectomy has become a mainstay of modern thoracic surgical practice and the technique of choice for resection of early-stage lung cancers. VATS procedures have distinct advantages to lung cancer patients over open surgery such as shorter hospitalization, reduced postoperative pain, and better cosmetic results (1). However, VATS is technically and physically demanding for the thoracic surgeon due to limitations in tissue manipulation and visualization as well as physical discomfort (2). More advanced VATS imaging systems and instrumentations have been developed to overcome many of the surgical disadvantages associated with VATS (3,4). Manipulation of the instruments is improved by the increased instrument range of motion, correction of the inverted instrument response, and reduction of human tremors.
Current thoracoscopic devices are not optimized to meet the unique challenges encountered with pulmonary vessel division in difficult-to-access locations within the pleural cavity. Hence, the ECHELON FLEX™ powered vascular stapler (PVS), was developed to address these issues by decreasing its width, adding a curved tip and narrower anvil, making the shaft slimmer and increasing articulation to enable increased precision for placement on the fragile pulmonary vasculature.
The ECHELON FLEX™ PVS demonstrated equivalent hemostatic performance compared to conventional staplers and improved access capability for pulmonary vascular procedures in vivo (5). However, there remains limited evidence available in literature and feasibility in clinical practice.
The current study aimed to assess the clinical effectiveness (requirement of intra-op intervention after firing) of ECHELON FLEX™ PVS for transection of the PA and PV during a VATS lobectomy.
Methods
Study population
This was a prospective, multi-center, post market clinical trial (NCT03056300) conducted under a single protocol at four high volume lung cancer institutions in China. The study was approved by the Independent Ethics Committee Review Board at each of the participating institutions. Patients were selected based on age (18 to 75 years), Eastern Cooperative Oncology Group performance status of 0 to 1, American Society of Anesthesiologists (ASA) score of 3 or less, no previous history of VATS or open lung surgery, and confirmed or suspected diagnosis of clinical stage IA to IIB non-small cell lung cancer (NSCLC) scheduled for a VATS lobectomy.
Patient exclusions were those who had an active bacterial or fungal infection, systemic usage of steroids, uncontrolled diabetes mellitus, end-stage renal or liver disease, severe cardiovascular disease, forced expiratory volume in one second <50%, severe chronic obstructive pulmonary disease, prior chemotherapy or radiation for lung cancer, concurrent surgical procedures other than diagnostic wedge resection followed by lobectomy, planned robotic-assisted procedure, pregnancy or lactating females at the time of screening, psychological conditions that would impair study participation, judged unsuitable for study participation by the site investigator, participant in any other investigational drug (within 30 days or 5 half-lives of an investigational drug) or device study; or compliance with follow-up visits and examinations. Written informed consent was obtained from all patients before initiation of any study procedures.
This study was conducted in accordance with the International Council for Harmonization (ICH) Guideline for Good Clinical Practice [1996], the current revision of the Declaration of Helsinki [2008], China Food and Drug Administration Regulations, Regulations on the supervision and administration of medical devices (State Council Decree No. 650), Guideline for Chinese Good Clinical Practice, as well as any other applicable local regulatory requirements.
Study design
The clinical effectiveness and safety of ECHELON FLEX™ PVS for VATS lobectomy were evaluated in this post market study. The primary effectiveness endpoint was the incidence of hemostatic interventions/procedures completed for intra-operative bleeding related to the transection of the pulmonary artery (PA)/pulmonary vein (PV) during VATS lobectomy. This was defined as intraoperative bleeding controlled using additional stapling, suture repair, clip placement, compression, sealant application, buttress, additional energy, increased transfusion requirements or additional surgical procedure. The primary safety endpoint was the incidence of hemostatic interventions/procedures completed for post-operative bleeding related to the transection of the PA/PV during VATS lobectomy defined as postoperative hemorrhage requiring additional blood products or surgical intervention. Adverse events (AEs) and serious adverse events (SAEs) were coded using the Medical Dictionary for Regulatory Activities (MedDRA).
Additional data collected in this study included measures of device usability and surgeon satisfaction. These included a surgeon satisfaction questionnaire, a surgeon device questionnaire, and the Surgeon Task Load Index (SURG-TLX). Device usability was assessed using questions specifically related to the articulation and the angle range of the device. The utility of ECHELON FLEX™ PVS was assessed using the SURG-TLX (6). The SURG-TLX allows surgeons to rate 6 specific components after each surgery performed, including mental fatigue, physical fatigue, hurried/rushed pace, procedure complexity, anxious while performing procedure, and distracting operating environment. Details of the intra-operative use of ECHELON FLEX™ PVS were assessed utilizing a surgeon device questionnaire with questions including alignment in relation to the vasculature compared to their current standard of care device, having the potential to reduce the amount of dissection compared to their current standard of care device, potential for surgeon stress reduction during PA/PV transection compared to their current standard of care device, and allowing for targeted vascular control compared to their current standard of care device.
Patient reported outcomes were reflected using the American Shoulder and Elbow Surgeons (ASES) Society standardized shoulder assessment form, and incisional and chest tube site pain measured by the Numerical Rating Scale (NRS) in both the perioperative period as well as at 4 weeks follow-up. The ASES assessment form is a patient-reported outcome designed to measure functional limitations and pain of the shoulder through assessment of basic tasks (i.e., activities of daily living), and is well characterized and accepted in the scientific community (7). The assessment utilizes 10 questions that are answered with unable to do =0, very difficult to do =1, somewhat difficult to do =2, and not difficult =3. Scores for each patient are obtained by adding the responses to the 10 questions, allowing for a maximum score of 30.
Statistical methods
The number and percentage of transections that required intervention was calculated and a 95% confidence interval was estimated. A similar summary was provided for the number and percentage of patients requiring post-operative interventions in relation to ECHELON FLEX™ PVS for PA or PV transection. Additional endpoints were summarized using descriptive statistics and 95% confidence intervals were calculated as appropriate for continuous or categorical measurements. Summary scores for the SURG-TLX provided an overall score which is calculated as the average of the six components for each surgery (6). Safety was assessed through the incidence of AEs and SAEs at the preferred term level, which were coded using MedDRA.
Results
Study population
Between January and May 2017, 50 patients were enrolled in the trial. There were 3 intra-operative withdrawals due to protocol exclusions. The patient demographic and surgical characteristics are presented in Table 1. The study population was 56.0% female, and all patients were Asian, with the majority being of Han Chinese ethnicity (94.0%). The mean duration of the procedure was 147.9 minutes, ranging from 54 to 485 minutes. The mean blood loss during the procedure was 60.9 mL, ranging from 10 to 400 mL. No patients required an intra-operative blood transfusion.
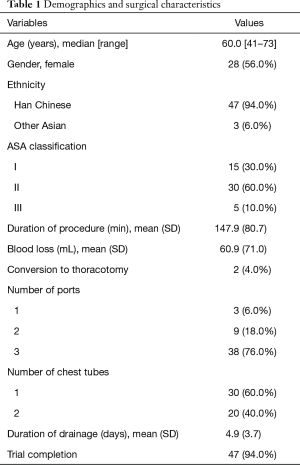
Full table
Clinical effectiveness and safety outcomes
A total of 167 PAs/PVs were transected with the majority (67.7%) being PAs. Hemostatic interventions were required in 3 (1.8%) of those transections [95% confidence interval (CI): 0.4% to 5.2%]; one case requiring suture repair, one case requiring additional stapling, and the remaining case requiring conversion to an open procedure (Table 2). No patients required hemostatic interventions or procedures for post-operative bleeding related to the transection of the PA/PV during VATS lobectomy.
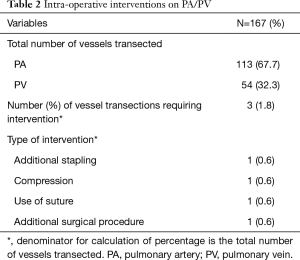
Full table
A total of 14 (28.0%) patients reported at least one AE during the study. The most common AEs reported were pyrexia in 5 (10.0%) patients, postoperative pneumothorax in 2 (4.0%) patients, and prolonged pulmonary air leak (>5 days) in 2 (4.0%) patients. Table 3 represents all AEs reported during the study. Twelve of the 14 patients rated their AEs mild to moderate while only 2 of the 14 patients had a severe rating. Furthermore, 5 patients reported SAEs including postoperative pneumothorax (n=2), acute myocardial infarction (n=1), chylothorax (n=1), and pulmonary hemorrhage (n=1). The case involving pulmonary hemorrhage was considered as having a possible relationship to the study device, however no other device-related AEs were reported during the study. Complete resolution of all SAEs was noted by study completion.
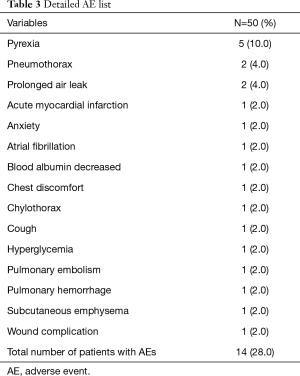
Full table
Surgeon satisfaction questionnaires
Device usability regarding articulation was surveyed in relation to the articulation feature after each surgical procedure in all 50 cases. Articulation was deemed essential to performing the surgery in 44 (88.0%) cases and was indicated as simplifying the surgery in all 50 cases. Surgeon satisfaction was also assessed after completion of each surgical procedure with 96.0% of cases being rated as satisfied or higher, 1 case (2.0%) rated as neutral and 1 case (2%) rated as dissatisfied. No tissue slippage was observed in 70.0% of cases and some tissue slippage was observed in 30.0% of cases. There were no cases of extensive tissue slippage observed in the study.
Surgeon device questionnaire
Intra-operative use of ECHELON FLEX™ PVS was assessed using a Surgeon Device Questionnaire. Figure 1 shows that, compared to their current standard of care device, in at least 78% of the cases, surgeons agreed (strongly or slightly) that ECHELON FLEX™ PVS enabled easier placement of the device on vessels, potential to reduce the amount of dissection required, potential to reduce the surgeon stress, and allowed for precise control and placement.
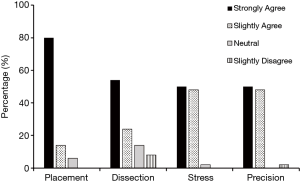
SURG-TLX
The SURG-TLX was used to evaluate additional stress introduced into the procedure through use of ECHELON FLEXTM PVS. Among the component scores, the operating environment was noted to be the main source of distraction with a mean (SD) score of 75.5 (25.0) based on a scale range of 0 to 100. Results from the other components of the SURG-TLX were within expectations with a mean score of ≤20.3.
Patient reported outcomes
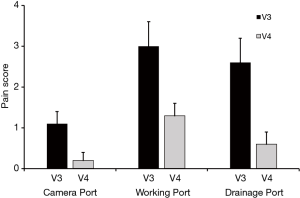
Patients were asked to rate post-operative pain on an NRS of 0 to 10 at three port sites (camera port, working port, and drainage port) at the time of discharge and approximately 4 weeks later. Figure 2 demonstrates that pain at all 3 port sites was generally tolerable (NRS 3.0 on average) at discharge and patients experienced near complete resolution at the 4-week follow-up (NRS 1.3 on average). The ASES assessment result demonstrated that patients had returned to baseline physical function by the 4-week follow-up visit (Figure 3). Complete details are included in the supplementary appendix online.

Discussion
This study reflects that the ECHELON FLEX™ PVS demonstrated effective surgical effectiveness in transecting PAs/PVs during VATS lobectomy while minimizing hemostatic intervention/procedures for intra-operative bleeding associated with transections. No hemostatic interventions or procedures related to vascular transection were needed during the postoperative period. Therefore, based on the safety data observed in this study, we concluded that the ECHELON FLEX™ PVS was safe. Additionally, other findings of this multi-center post market study showed that the ergonomic design of ECHELON FLEX™ PVS is feasible for VATS lobectomy in the Asian patient population and may convey a variety of multifactorial benefits to both the surgeons and patients. The majority of surgeons participating in the study were satisfied with the overall usability of ECHELON FLEX™ PVS. The feasibility assessment of using ECHELON FLEX™ PVS during VATS lobectomy reinforces the advantages of the device that have been demonstrated in an earlier study (8).
Managing the safety of patients undergoing VATS lobectomy requires an understanding of instrumentation and environmental factors related to surgical care in addition to the physiological and psychological characteristics that contribute to medical error and iatrogenic injury. Of the 167 PAs/PVs that were transected, intra-operative bleeding related to the transection of the PA/PV during VATS lobectomy occurred in 3 (1.8%) transections. The occurrence of AEs was consistent with VATS lobectomy (9), with a total of 14 (28.0%) patients reporting at least 1 AE, the most common being postoperative pyrexia in 5 (10.0%) patients, postoperative pneumothorax and prolonged air leak each occurring in 2 (4.0%) patients. One event of pulmonary hemorrhage was considered related to the study device and a total of 5 SAEs were reported.
Pain scores were collected at three port sites (camera port, working port, and drainage port) at the time of discharge and approximately 4 weeks later. At discharge, the pain was mild on average (average NRS scores ranging from 1.1 to 3.0). Although the working port score was the highest observed in this data collection (mean 3.0 at discharge), this score in the context of the entire scale was considered mild (1–3 scores considered mild, 4–6 scores moderate, and 7–10 scores severe). By week 4, the average scores for all three sites were at 1.3 or below, which is considered a mild pain score (10). For the ASES Assessment of shoulder pain, both the right and left shoulder scores started close to the maximum score of 30 at the pre-surgery timepoint. At the post-op through discharge visit, there were decreases in both shoulders, and then by the 4-week follow-up timepoint, both shoulders had almost returned to pre-surgery values.
Reduction of the burden on operating theater personnel has gained interest recently (2), and can contribute to maintaining health of surgeons. The increased risk for work-related musculoskeletal disorders is associated with the lengthy overload of fine-hand motor manipulation. There is a growing literature on the effects of work-related fatigue in healthcare personnel associated with musculoskeletal disorders pain and injuries (11,12). When assessing surgeon satisfaction, ECHELON FLEX™ PVS delivered on its goal of placement, with approximately 80% of surgeons strongly agreeing that ECHELON FLEX™ PVS enabled easier placement compared to their current standard of care. When evaluating the SURG-TLX, the device did not contribute to the surgeon’s workload, it was predominantly driven by the operating environment itself with the distracting operating environment component being the only component outside of the “Low” perceived workload grade of the scale (0–50). Overall, the advanced design feature of the device and size (e.g., narrow anvil of 7 mm, curved dissection tip, slim shaft of 9 mm in diameter) and clinical benefits (e.g., easy access in restricted narrow space) resulted in easy placement of the device and was applicable to areas with limited access during the procedure.
This study has several limitations including lack of a comparator group or control arm as well as procedure technique variability given the practical and logistical challenges of implementing a uniform surgical procedure between institutions. Additionally, potential for selection bias could be a limitation as surgeons were at their discretion to enroll patients with different anthropometrics in the current trial. This detracts from the homogeneity of the group, although this aspect may be more representative of a “real world” Chinese lung cancer cohort. Finally, learning curves among different surgeons between institutions for ECHELON FLEX™ PVS could potentially be a confounding factor.
Conclusions
For VATS lobectomy, ECHELON FLEX™ PVS demonstrated effective surgical effectiveness as well as value in cognitive and physical distress reduction. Complications following VATS lobectomy to treat NSCLC were generally low as expected. Few patients required a hemostatic intervention/procedure for intra-operative bleeding related to the transection of the PA and PV during VATS lobectomy. In addition, there were favorable results reported by the surgeons on the surgeon satisfaction questionnaire regarding usability and surgeon stress. The results showed a low stress load and increase in work efficiency which supported the surgeons’ preference for ECHELON FLEX™ PVS.
Acknowledgments
This study was funded by Ethicon Endo Surgery, Inc. and Johnson & Johnson Medical (Shanghai) Ltd.
Footnote
Conflicts of Interest: B Qiu, X Kang, KN Chen, J Hu, J Li, L Zheng, and S Gao declared that they were funded for their participation. D Ding and T Yang are employed by Johnson & Johnson Medical (Shanghai) LTD. EJ Fegelman, ML Schwiers, EE Creedon and JR Waggoner are employed by Ethicon, Inc.
Ethical Statement: The study was approved the Ethics Committee at each institution and informed consent was obtained from all patients.
References
- Berfield KS, Farjah F, Mulligan MS. Video Assisted Thoracoscopic Lobectomy for Lung Cancer. Ann Thorac Surg 2019;107:603-9. [Crossref] [PubMed]
- Epstein S, Sparer EH, Tran BN, et al. Prevalence of Work-Related Musculoskeletal Disorders Among Surgeons and Interventionalists: A Systematic Review and Meta-analysis. JAMA Surg 2018;153:e174947. [Crossref] [PubMed]
- Özyurtkan MO, Kaba E, Toker A. Technological innovation in video-assisted thoracic surgery. J Vis Surg 2017;3:20. [Crossref] [PubMed]
- Miller DL, Roy S, Kassis ES, et al. Impact of Powered and Tissue-Specific Endoscopic Stapling Technology on Clinical and Economic Outcomes of Video-Assisted Thoracic Surgery Lobectomy Procedures: A Retrospective, Observational Study. Adv Ther 2018;35:707-23. [Crossref] [PubMed]
- Ng CS, Pickens A, Siegel JM, et al. A novel narrow profile articulating powered vascular stapler provides superior access and haemostasis equivalent to conventional devices. Eur J Cardiothorac Surg 2016;49 Suppl 1:i73-78. [PubMed]
- Wilson MR, Poolton JM, Malhotra N, et al. Development and validation of a surgical workload measure: the surgery task load index (SURG-TLX). World J Surg 2011;35:1961-9. [Crossref] [PubMed]
- Richards RR, An KN, Bigliani LU, et al. A standardized method for the assessment of shoulder function. J Shoulder Elbow Surg 1994;3:347-52. [Crossref] [PubMed]
- Kuroda H, Yoshida T, Sakao Y. A powered vascular staple for the application of segmental bronchial closure in thoracoscopic anatomic segmentectomy. J Thorac Dis 2017;9:5352-4. [Crossref] [PubMed]
- Yang J, Xia Y, Yang Y, et al. Risk factors for major adverse events of video-assisted thoracic surgery lobectomy for lung cancer. Int J Med Sci 2014;11:863-9. [Crossref] [PubMed]
- McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. Mosby, St. Louis: Mosby, 1989.
- Davis KG, Kotowski SE. Prevalence of Musculoskeletal Disorders for Nurses in Hospitals, Long-Term Care Facilities, and Home Health Care: A Comprehensive Review. Hum Factors 2015;57:754-92. [Crossref] [PubMed]
- Occhionero V, Korpinen L, Gobba F. Upper limb musculoskeletal disorders in healthcare personnel. Ergonomics 2014;57:1166-91. [Crossref] [PubMed]


