Immunosuppression in lung transplantation
Lung transplantation can be a life-saving procedure for those with end-stage lung diseases. Unfortunately, long term graft and patient survival are limited by both acute and chronic allograft rejection, with a median survival of just over 6 years (1). Immunosuppressive regimens are employed to reduce the rate of rejection, and while protocols vary from center to center, conventional maintenance therapy consists of triple drug therapy with a calcineurin inhibitor (cyclosporine or tacrolimus), antiproliferative agent [azathioprine (AZA), mycophenolate, sirolimus (srl), everolimus (evl)], and corticosteroids (CS). Roughly 50% of lung transplant centers also utilize induction therapy, with polyclonal antibody preparations [equine or rabbit anti-thymocyte globulin (ATG)], interleukin 2 receptor antagonists (IL2RAs) (daclizumab or basiliximab), or alemtuzumab (2). While these agents are used to prevent acute and chronic rejection, they are not without adverse effects, including drug-specific toxicities, as well as opportunistic infections and malignancy. This review will summarize these agents and the data surrounding their use in lung transplantation, as well as additional common and novel therapies in lung transplantation.
Induction immunosuppression
Induction therapy is intensive immunosuppressant therapy given perioperatively to reduce the risk of acute rejection and also serves to delay initiation of maintenance immunosuppression, most notably the nephrotoxic calcineurin inhibitors. These agents primarily target T lymphocytes, which are considered the effector cells in cell-mediated rejection.
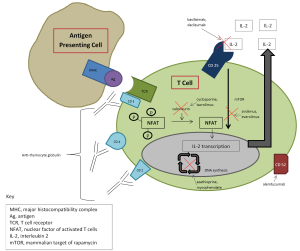
According to the most recent registry report of the International Society for Heart and Lung Transplantation (ISHLT), of the centers that utilize induction, majority use an IL2RA (2). Both daclizumab and basilixmab are non-depleting monoclonal antibodies that bind to the alpha subunit of the interleukin 2 (IL-2) receptor (CD25) present on activated T lymphocytes, thereby preventing T cell activation and proliferation (3,4). Daclizumab is a humanized (90% human, 10% murine) (3) monoclonal antibodythat was removed from the US market in 2009 (FDA), thus making basiliximab the only IL2RA available for use. Basiliximab is a chimeric (75% human, 25% murine) monoclonal antibody and is generally well tolerated, with adverse effects similar to that of placebo (4). ATG is the second most commonly used induction agent, used by roughly 20% of centers that utilize induction (2). ATG is a polyconal antibody preparation isolated from either rabbit (rATG, Thymoglobulin©) or horse (equine ATG, ATGAM©) sera which contain antibodies toward human thymocytes and cause significant T cell depletion (5,6). Adverse effects associated with these agents include fever, chills, rash, arthralgia, diarrhea, leukopenia, and thrombocytopenia. Pre-medication with acetaminophen, anti-histamines, and CS are usually required and help minimize these reactions. Serum sickness and anaphylaxis have also been reported, in addition to increased rates of infection and malignancy.
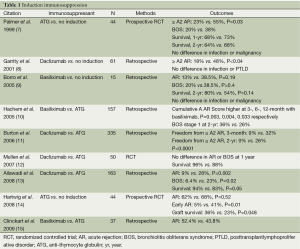
Data for the use of induction in lung transplantation are presented in Table 1. Overall it appears that induction with either ATG or an IL2RA reduces or delays the incidence of acute rejection, bronchiolitis obliterans syndrome (BOS), and may improve graft and patient survival compared to no induction (7-9,14). Studies comparing IL2RAs and ATG show inconclusive results; one study indicated IL2RAs are associated with lower rates of acute rejection and BOS, as well as improved survival (13); three studies showed lower acute rejection and BOS and improved survival with ATG (10,11,15), while still another showed no difference (12). In 2008, Hachem and colleagues published a registry report that retrospectively analyzed 3,970 adult lung transplant recipients. Four year graft survival in those who received induction with an IL2RA, ATG, or no induction were 64%, 60%, and 57% (P=0.0067), respectively (16). Reasons for such variability in outcomes relate to the size and retrospective nature of these studies, potential differences in patient population and management, duration of followup, and variability in maintenance immunosuppression regimens. More recently, alemtuzumab, a humanized monoclonal antibody targeting CD52, has been used as an induction agent. The CD52 antigen is found on T and B lymphocytes, as well as natural killer cells, monocytes and macrophages (17). Upon binding, alemtuzumab induces cellular lysis and causes significant and prolonged depletion, with B cell recovery occurring within 3-6 months and T cell recovery >12 months (18,19). This profound and prolonged lymphocyte depletion associated with alemtuzumab may allow for the possibility of reduced maintenance immunosuppression. Loenhout and colleagues published their findings using alemtuzumab induction in 20 lung transplant recipients with reduced maintenance immunosuppression in 2010. Compared to 20 historical controls who received standard maintenance immunsuppression, there were no statistical differences between 6- or 12-month survival (95% vs. 90%, 76% vs. 95%), episodes of acute rejection (2/16 vs. 5/20), or bacterial, viral or fungal infections (20). Subsequently, Shyu and colleagues published 5 year outcomes using alemtuzumab induction with reduced-intensity maintenance immunosuppression. Their retrospective analysis grouped patients according to induction type: alemtuzumab (n=127), ATG (n=43), daclizumab (n=73), or none (n=93). Graft survival differed by group: 59%, 44%, 41%, 47%, respectively; as did freedom from acute rejection: 30%, 20%, 19%, 18%, respectively; freedom from lymphocytic bronchiolitis: 82%, 54%, 55%, 70% respectively; and freedom from BOS: 54%, 27%, 43%, 46% respectively (21). While alemtuzumab induction with reduced maintenance immunosuppression thus far demonstrates similar if not improved overall outcomes compared to other induction regimens, the optimal induction and maintenance regimen still needs to be elucidated by large, randomized controlled trials. Though 50% of centers currently utilize induction, enhanced immunosuppression must be weighed against adverse effects, including infection and malignancy. Large, randomized controlled trials measuring the difference in acute rejection, BOS, graft and patient survival, infection and malignancy comparing no induction, IL2RAs, ATG, and alemtuzumab are needed to better understand the effect of the agents and to identify the optimal regimen for lung transplant recipients.
Full table
Maintenance immunosuppression
Maintenance immunosuppression is lifelong immunosuppressive therapy that is given to prevent both acute and chronic rejection. The goal is to not only to prevent and minimize immune-mediated injury to the allograft but also to minimize adverse effects associated with the medications used. Conventional maintenance immunosuppressive regimens consist of triple drug therapy with a calcineurin inhibitor, antiproliferative agent, and CS. Historically cyclosporine and AZA were used along with prednisone, but over time additional agents have emerged on the market, including tacrolimus, mycophenolate, and the mammalian target of rapamycin (mTOR) inhibitors, srl and evl. Despite the addition of these agents to the armamentarium of immunosuppression for lung transplant recipients, acute rejection and BOS remain obstacles to long-term survival. Additionally, minimization and management of adverse effects continuesto be challenging. Selection of regimens is largely protocolized and based on studies from other types of organ transplantation as well as currently available literature in lung transplant, and center-specific outcomes and provider experience.
Calcineurin inhibitors
Cyclosporine was the first calcineurin inhibitor available for use, first approved by the FDA in 1983. It is a lipophilic compound that binds to intracellular cyclophilin in T lymphocytes, forming a complex that prevents transcription of interleukin 2, thereby decreasing activation and proliferation of T lymphocytes (22). Oral absorption of cyclosporine (Sandimmune©) is poor and variable (10-89%). A modified cyclosporine formulation was subsequently developed and approved by the FDA in 1997 (Neoral©) with enhanced bioavailability, with approximately 50-150% increases in area under the curve (AUC) and Cmax (23,24). Sandimmune and Neoral are not interchangeable but both are available in capsules, oral solution, and intravenous formulations. Therapeutic drug monitoring of cyclosporine consists of measuring trough (C0) values, AUC calculations, or 2-hour post-dose (C2) levels. In renal transplantation, AUC measurements have demonstrated superiority over troughs (25), however this requires multiple samples to estimate AUC, which is time consuming, cumbersome and impractical. A limited sampling strategy (LSS) may be employed as an alternative, measuring 2 post-dose levels (26), but this method still requires multiple samples and a calculation to estimate AUC. Therefore most centers utilize either C0 or C2 levels. Studies in lung transplant recipients indicate that C2 is a better correlate with AUC than C0 (27) and may reduce short-term nephrotoxicity associated with cyclosporine compared with C0, without compromising lung function (28). Target ranges vary according to center-specific protocols and practices, and take into account patient characteristics, such as time post-transplant and rejection and infection history. Generally, target trough levels range from 100-450 ng/mL, or C2 levels 800-1,400 ng/mL. Major adverse effects of cyclosporine include nephrotoxicity (acute and chronic), hypertension, hypercholesterolemia, electrolyte abnormalities (hyperkalemia, hypomagnesemia), neurotoxicity (posterior reversible encephalopathic syndrome, seizures, headache, tremor), diabetes, hirsutism, and gingival hyperplasia. A second calcineurin inhibitor, tacrolimus(previously known as FK506) (Prograf©) became available for use in 1997. It is 10-100 times more potent than cyclosporine. Tacrolimus binds to intracellular FKBP12, forming a complex that prevents transcription of cytokines, including interleukin 2, and ultimately prevents T lymphocyte activation and proliferation (29). Like cyclosporine, tacrolimus has poor and variable absorption, 17-23% (29). Tacrolimus is available in oral capsules and as an intravenous formulation. There is no commercially available oral suspension however formulas for pharmaceutical compounding are available. Sublingual administration of tacrolimus capsules at half of the oral dose is an option for those who are unable to tolerate oral therapy and wish to avoid intravenous tacrolimus due to significant toxicity (30). A once-daily extended-release formulation of tacrolimus, marketed under the trade name Astragraf XL® was approved by the FDA in 2013. No studies have yet been performed in lung transplant recipients; however they may be available in the future. Despite multiple studies indicating post-dose levels to more accurately predict AUC, most centers utilize trough concentrations for therapeutic drug monitoring (31,32). Target ranges vary according to center-specific protocols and practices, and take into account patient characteristics, such as time post-transplant and rejection and infection history. Generally, target trough concentrations range from 5-15 ng/mL. Tacrolimus displays similar adverse effects to cyclosporine, with perhaps less hypertension and hypercholesterolemia, but more neurotoxicity and diabetes (33-39). Thrombotic thrombocytopenia purpura and hemolytic uremic syndrome have been reported with both cyclosporine and tacrolimus (40). Both cyclosporine and tacrolimus undergo metabolism via the hepatic cytochrome (CYP) P450 3A4 and 3A5 enzymes and p-glycoprotein efflux pumps present on intestinal mucosa,leading to significant drug interactions with CYP inducers (e.g., rifampin, phenobarbital, carbamazepine, phenytoin) and inhibitors (e.g., azoles, macrolides, calcium channel blockers). Additional drug interactions exist for cyclosporine, as it is not only a substrate of CYP 3A4 but also a moderate inhibitor (statins).
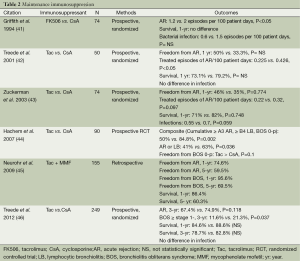
Selected data comparing cyclosporine and tacrolimus are shown in Table 2. Majority of the trials are small, prospective, randomized studies showing no statistical differences in acute rejection or survival between those treated with cyclosporine or tacrolimus, whether receiving no induction or ATG, AZA or mycophenolate. The most recent study published in 2012 by Treede et al. is the largest study to date and showed no difference between cyclosporine and tacrolimus in acute rejection or survival at 3-year, however there was a higher incidence of BOS stage 1 or greater with cyclosporine and it was also shown to be a risk factor for the development of BOS by univariate analysis (46). According to the most recent ISHLT Registry report, tacrolimus was the most frequently used calcineurin inhibitor, 83% at one year post-transplant, 77% at 5 years post-transplant (2).
Full table
Anti-proliferative agents
AZA was the first anti-proliferative agent available for use. AZA is converted to 6-mercaptopurine (6-MP) in vivo which then is converted into several compounds that get incorporated into the DNA of replicating cells and halt proliferation (47). AZA is associated with significant leukopenia, thrombocytopenia, anemia, hepatotoxicity (transaminitis and cholestasis), and rarely pancreatitis. Caution must be used when using AZA with xanthine oxidase (XO) inhibitors (e.g., allopurinol). XO is thought to be responsible for converting 6-MP to metabolites. The combination results in significant bone marrow suppression and a 75% dose reduction of AZA in combination with XO inhibitors is generally recommended. The typical starting dose is 2 mg/kg IV or orally daily.
Mycophenolate is the most frequently used antiproliferative agent used according to the most recent ISHLT Registry report (2). Mycophenolate mofetil and mycophenolate sodium are converted to the active metabolite, mycophenolic acid (MPA), which inhibits inosine monophosphate dehydrogenase (IMPDH), the enzyme responsible for T and B lymphocyte production. Inhibiting this enzyme results in decreased T and B lymphocyte proliferation. Because lymphocytes lack the ability to utilize salvage pathways for nucleotide synthesis and thus rely on the IMPDH pathway, mycophenolate is selective for T and B lymphocyte proliferation inhibition (47). Mycophenolate undergoes rapid absorption and conversion to MPA. MPA is metabolized hepatically into mycophenolic acid glucuronide (MPAG). MPAG is excreted via bile into the intestines, where it is converted back to the active metabolite, MPA, resulting in a second peak concentration in the plasma. Doses range from 1-1.5 g IV or oral twice daily. Therapeutic drug monitoring is available for mycophenolate, with AUC being the optimal parameter for measuring treatment response. Trough values have shown poor predictive response (48-50). LSS calculations for estimation of AUC in lung transplant patients are also availablehowever therapeutic drug monitoring has not been firmly established (51). Principle adverse effects of mycophenolate are leukopenia, thrombocytopenia, and gastrointestinal disturbances (diarrhea, abdominal pain, nausea, vomiting). Initial use of mycophenolate involved rescue therapy following development of BOS, with stabilization of pulmonary function testing after switching from AZA (52). In a prospective, randomized trial of 81 lung transplant recipients comparing azathiopine to mycophenolate in combination with cyclosporine and CS, there were no differences in biopsy-proven or clinical rejection, survival, infection, or adverse drug events at 6-month (53). A subsequent prospective, randomized multicenter study comprising 315 lung transplant recipients also showed no difference between AZA and mycophenolate when used in combination with cyclosporine and CS in the outcomes of acute rejection, BOS, and survival at 3-year, however a greater percentage of patients discontinued AZA than mycophenolate (59.6% vs. 46.5%) (54).
Srl and evl are two newer antiproliferatives in the mTOR inhibitor class. Both bind to intracellular immunophilin FK506 binding protein like tacrolimus, however unlike tacrolimus the complexes they form do not inhibit calcineurin but instead bind to mTOR, which is a signaling pathway needed to promote progression of the cell cycle from G1 to S phase. The end effect of mTOR inhibitors is a decrease in T lymphocyte activation and proliferation (47). Srl is available as oral tablets and an oral solution. Doses range from 0.5-6 mg daily, with target trough values ranging 5-15 ng/mL. Evl is available as oral tablets. Doses range from 0.25-3 mg twice daily, with target trough values ranging 5-15 ng/mL. Notable adverse effects include decreased wound healing, leukopenia, thrombocytopenia, hypertriglyceridemia, proteinuria, and pneumonitis. Both are metabolized by CYP 3A4 and therefore have similar drug interactions as tacrolimus. The role of mTOR inhibitors in lung transplant is still being identified. They may be used in conjunction with or substituted for either calcineurin inhibitors or other antiproliferative agents. The most common reasons for use include kidney dysfunction due to calcineurin inhibitors, onset of BOS, and malignancy (55-57). For those who exhibit kidney dysfunction, adding an mTOR inhibitor and reducing the calcineurin inhibitor dose has been shown to improve kidney function (55,58,59).Additionally, due to their antiproliferative and anti-fibroblast effects (60), mTOR inhibitors have been used in lung transplant recipients with BOS to help slow progression. Indeed small, retrospective studies have shown stabilization or improvement in pulmonary function testing in lung transplant recipients with BOS (55,56,61,62). Two studies used srl immediately post-transplant and reported significant wound dehiscence and airway complications, leading to death in some patients (63,64), so mTOR inhibitors should not be used until the anastomosis and airways have healed. In 2006, Snell and colleagues performed a prospective randomized controlled trial comparing AZA and 3th month conversion to evl in 213 lung transplant recipients also maintained on cyclosporine and CS. The composite endpoint of efficacy failure (>15% FEV1 decline from baseline, graft loss, death or loss to follow up) occurred in 33.9% vs. 21.8% of patients at 12-month (P=0.046), however there was no difference in this composite endpoint at 24-month. The authors concluded that evl did demonstrate a slowing in loss of pulmonary function over time (65). Most recently, Sacher and colleagues published data on 24 lung transplant recipients who were converted to srl prophylactically vs. AZA/MMF, one year post-transplant. Of the 19 patients who remained on long-term srl, a trend toward a reduction in the incidence of BOS and improved survival was reported (66). Larger, randomized controlled trials are needed to more fully elucidate the effect of mTOR inhibitors in the prevention of BOS.
Corticosteroids (CS)
CS have been used in solid organ transplant since the very beginning and have not only remained a corner stone of both induction and maintenance immunosuppression but they are also used to treat acute cellular rejection (ACR) as well. The most commonly used CS in solid organ transplant are methylprednisolone and prednisone. CS are known to have antiinflammatory properties and exert their effects in a variety of ways, including inhibiting the NFkB pathway, preventing T cell proliferation, decreasing macrophage activation, inhibitingcytokine production and altering lymphocyte migration (67). According to the most recent ISHLT registry report, CS continue to be used by almost all transplant centers, at one and five years post-transplant. Initial doses range from 500-1,000 mg given intraoperatively, and are gradually tapered over weeks to months to 5-10 mg per day for maintenance. Short and long term use of CS is associated with significantside effects, including hypertension, weight gain, hyperlipidemia, hyperglycemia and diabetes mellitus, osteoporosis and increased risk of fractures, increased risk of cataracts, poor wound healing, psychiatric disturbances and infectious complications.Data on steroid-free regimens in lung transplantation is lacking and at best shows limited success (68,69). Complete steroid-withdrawal should be avoided at the present time, owing to a significant risk of allograft dysfunction; however, doses should be lowered as quickly and as safely as possible, and maintainthe lowest possible doses with the goal of stable and optimal lung function while avoiding and minimizing drug-related adverse effects (Figure 1).
Antihumoral therapy
Generally immunosuppression is employed to suppress cell mediated immunity by targeting T cell function and proliferation as rejection is usually a cell mediated phenomenon. However the role of humoral or antibody-mediated rejection (AMR) in solid organ transplant recipients has become more evident over the years. Antibody mediated rejection has been identified and characterized in other organs but remains poorly defined in lung transplant recipients. No agreed upon pathologic criteria exists to date in lung transplantation (70,71). Mechanisms by which anti bodies, which usually are donor specific antibodies (DSA), produce injury are not yet well described. Injury may be complement mediated or complement independent (72). No universally agreed upon management strategy exists for these antibodies. Use of intra venous immunoglobulin (IVIG), one of most commonly used treatments with a relatively low side effect profile, with or without plasmapheresis, peritransplant and after development of DSA post-transplant resulted in improvement in certain parameters such as acute rejection and BOS at a single institution (73). In a study reported by Hachem and colleagues, use of IVIG combined with rituximab, a monoclonal anti CD20 antibody, vs. IVIG to clear newly acquired DSA showed improved survival and freedom from BOS in patients who cleared DSA after treatment. However there was no improvement in clearance of DSA with addition of rituximab to IVIG (74). Plasmapheresis is mainly used for antibody removal from circulation in suspected cases of humoral rejection which do not respond to steroids, leading to clinical improvement (75). Bortezomib, an inhibitor of 26S proteasome that leads to plasma cell apoptosis, has been used successfully in case reports to treat possible acute humoral rejection in lung transplant recipients (76,77). Hyperacute rejection due to pre formed antibodies against donor HLA antigens has become uncommon due to ongoing cross match screening. Treatment with IVIG, plasmaphresis, rituximab, antithymocyte globulin and eculizumab has been described in various case reports with variable degree of success (78-80).
Novel approaches
Aerosolized calcineurin inhibitors
A number of reports have been published regarding the use of aerosolized cyclosporine. In 1996, Iacono and colleagues published a report of histologic improvement of obliterative bronchiolitis (OB) and stabilization of pulmonary function testing in 7 lung transplant recipients who received aerosolized cyclosporine as rescue therapy (81). Shortly thereafter, the use of aerosolized cyclosporine to treat refractory acute rejection in 9 lung transplant recipients was associated with histologic improvement in 8 of 9 subjects, improvement in pulmonary function testing, a reduction in cycles of pulse dose CS and ATG, reduction in oral prednisone dose, and reduction in episodes of pneumonia was also observed, compared to 22 historical controls (82). Both reports showed no additional renal or hepatic toxicity with the use of aerosolized cyclosporine. A larger case-control study was subsequently undertaken and demonstrated a survival advantage in lung transplant recipients with biopsy-documented OB compared to conventional immunosuppression (83). While the most well-studied randomized placebo-controlled trial of aerosolized cyclosporine did not show a reduction in the primary endpoint of rate of ACR, it also demonstrated a survival advantage compared with conventional immunosuppression, and showed an improvement in chronic rejection-free survival (84). Despite these results, an FDA-approved formulation of aerosolized cyclosporine is still currently unavailable. Animal studies aiming to characterize aerosolized tacrolimus pharmacokinetics and safety have been published (85-87). The first case report of using tacrolimus via inhalation in a human lung transplant recipient with BOS was recently published demonstrating improved functional capacity and oxygenation after one week of therapy (88). More data are needed to determine the optimal use of aerosolized calcineurin inhibitors but this therapeutic approach seems promising.
Azithromycin
Azithromycin is a macrolide antibiotic with anti-inflammatory and immunomodulatory effects (89). These effects, in conjunction with the beneficial effects of maintenance azithromycin seen in cystic fibrosis patients led to pilot studies of azithromycin in lung transplant recipients with BOS (90-93). In 5 of 6 patients, thrice-weekly azithromycin for 13 weeks demonstrated an average 17% improvement in FEV1 (92) and an average 18% improvement in FEV1 after 12 weeks of therapy in 8 others (93). A retrospective analysis of 20 lung transplant recipients also demonstrated an improvement in FEV1 after 12 weeks of azithromycin therapy (average 110 mL from baseline) (94). However, not all patients respond to azithromycin therapy (95-97). Evidence suggests airway neutrophilia and elevated interleukin-8 bronchoalveolar (BAL) concentration may be predictors of response (95,97,98). Furthermore, studies have indicated that early initiation of azithromycin, e.g., BOS 0-p, may have more of an impact on preventing disease progression and may improve survival (97,99,100). In a randomized, placebo-controlled trial of 83 lung transplant recipients, there was a significant reduction in the incidence of BOS at 2-year in those who received azithromycin prophylactically compared to those who did not (12.5% vs. 44.2%, P=0.0017) (101). There was also a significant difference in BOS-free survival (HR 0.27, P=0.020), although overall survival was similar between groups. Collectively these data suggest early initiation of azithromycin in lung transplant recipients may prevent the incidence of BOS and prolong BOS-free survival, and may improve or stabilize pulmonary function after the onset of BOS, particularly in those with neutrophil- and IL-8-predominant BAL.
Extracorporeal photopheresis (ECP)
ECP was developed initially for treatment of cutaneous T cell lymphomabut has been utilized in variety of disease states including solid organ transplantation. The process involves leukopheresis followed by incubation of the isolated cells with 8-methoxypsoralen (8-MOP) and subsequent activation of 8-MOP with ultraviolet A radiation. These cells are then reinfused into the patient. 8-MOP activation causes DNA cross linkage and apoptosis. Reinfusion of these apoptotic cells generate T regulatory cells (T regs) and increased production of IL-10 and transforming growth factor beta. Exact mechanisms by which these immunomodulatory effects are produced are not well understood. At present, clinical studies assessing efficacy of ECP in lung transplant recipients are limited to retrospective single center studies done in patients showing declining lung function. No trials to assess the prophylactic effect of ECP on development of BOS by starting ECP immediately post-transplant have been done to date. In a study by Morrell and colleagues, 60 lung transplant patients received ECP in addition to conventional immunosuppression for treatment of progressive BOS. Fifteen patients (25%) showed an improvement in FEV1 and rest showed a reduction in rate of decline in FEV1 which persisted at 12 months after initiation of ECP (102). Another study done by Jaksch and colleagues, 51 lung transplant recipients who developed BOS and did not respond to augmentation of immunosuppression and azithromycin, received ECP.Thirty-one patients (61%) showed improvement or stabilization of lung function while 20 patients (39%) had continued decline in lung function and did not respond to ECP. Survival rate after start of BOS at 1, 3 and 5 years was significantly better in treatment responsive group (103).These studies did not identify any significant characteristics among lung transplant recipients that could predict the response to ECP. Recently a retrospective single center study done by Greer and colleagues assessed clinical efficacy of ECP treatment in lung transplant recipients with azithromycin-refractory chronic lung allograft dysfunction (CLAD) and attempted to associate clinical response to several CLAD phenotypes. Sixty-five lung transplant recipients were diagnosed and graded for graft dysfunction in accordance with ISHLT BOS criteria and were started on ECP treatment while showing deterioration or no improvement despite taking azithromycin which was started after reversible causes of graft dysfunction were excluded. Thirty-five patients (54%) showed improvement or stabilization of FEV1 while 30 patients showed >10% decline in FEV1. Three CLAD phenotypes, restrictive allograft syndrome, defined by TLC ≤90% of baseline, non neutrophilic CLAD, patients demonstrating BAL neutrophilia <15% and rapid decliners, patients suffering a >100 mL/month decline in FEV1 before ECP initiation showed that they were less likely to benefit from ECP treatment. Significant survival benefit was noted in the ECP responsive group when compared to the ECP refractory group (104). Randomized clinical trials are needed to better evaluate the benefit and possibility of early use of ECP after onset of CLAD in lung transplant recipients.
Statins
Statins, 3-hydroxy-3-methylglutaryl coenzyme Areductase inhibitors, have been shown to have properties which may have a potential beneficial impact on lung allograft function post-transplant. They have been shown to reduce the gamma interferon induced expression of major histocompatibility molecules on cells, increase the number of CD4+CD25+ T regs, inhibit growth factor expression in lung fibroblasts and inhibit the development of obliterative airway disease in animal models (105-108).
These abovementioned immunomodulatory and anti-fibroproliferative properties have potential benefit for lung transplant recipients. However, clinical evidence in lung transplant recipients is limited to retrospective single center studies only. Johnson and colleagues showed improved 6-year survival in statin group compared to controls, 91% vs. 54%, as well as reduced rates of acute rejection and BOS (109). Li and colleagues showed improved survival and maintenance of lung function associated with post-transplant use of simvastatin in a single center cohort analysis of 502 lung transplant recipients (110). Prospective randomized trials are needed to confirm these findings, compare different statins and determine the optimal dose.
Pirfenidone
Pirfenidone is an anti-fibrotic agent used to treat pulmonary fibrosis. It inhibits growth-factor dependent proliferation of fibroblasts, T cell proliferation and activation, and may inhibit dendritic cell activation and function (111-115), and may be a potential therapeutic strategy for the treatment of CLAD. Thus far two case reports of pirfenidone use in human lung transplant have been published (116,117). The first reported a mild increase in FEV1 following progressive decline with no evidence of infection or rejection and failure to respond to azithromycin, montelukast and fundoplication (116). The second reported a slower rate of decline in forced vital capacity, FEV1, and a mild increase in total lung capacity in a lung transplant recipient with restrictive allograft syndrome (117). Given these findings, further study of pirfenidone in human lung transplantation is warranted.
Treatment
ACR, AMR and CLAD are discussed in-depth elsewhere. Specific treatment protocols vary from center to center, but options are limited to high-dose or “pulse” CS (e.g., methylprednisolone 10-15 mg/kg IV daily × 3-5 days), particularly for initial treatment or minimal-mild grade ACR; ATG (1.5 mg/kg IV daily × 3-5 days) or alemtuzumab (30 mg IV once) for moderate-severe grade ACR or steroid-resistant/steroid-refractory ACR. Therapies available for treatment of AMR include plasmaphereis (5-6 cycles), IVIG (1-2 g/kg over 3-6 days), rituximab (375 mg/m2 IV weekly × 4 doses or 1,000 mg IV every 2 weeks × 2 doses), and/or bortezomib (1-1.3 mg/m2 every 72 hours × 4 doses). Treatment options for CLAD are even more limited, and there is currently no agent available to date that reverses that process and restores lung function, other than re-transplant when available. Therapies targeting the processes of CLAD either prevent the onset of CLAD, or prevent and delay its progression. These include azithromycin, ECP, the statins, and pirfenidone. Augmentation of immunosuppression with ATG, alemtuzumab, addition or substitution of an mTOR inhibitor to the maintenance regimen, substitution of mycophenolate for AZA or of tacrolimus for cyclosporine, are additional strategies that have been employed with varying success (Table 3).

Full table
Summary
Our understanding of the underlying mechanisms and clinical presentation of acute allograft rejection and CLAD continue to evolve. Immunosuppressive regimens have significantly contributed to the improvement of the survival of lung transplant recipients. Despite the progress in the management of lung transplant recipients, they continue to be at high risk of treatment-related complications, poor allograft and patient survival. Randomized clinical trials are needed to allow the development of better agents, regimens and techniques to address above mentioned issues and reduce morbidity and mortality among lung transplant recipients.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: thirtieth adult lung and heart-lung transplant report--2013; focus theme: age. International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2013;32:965-78. [PubMed]
- Christie JD, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. International Society of Heart and Lung Transplantation. J Heart Lung Transplant 2012;31:1073-86. [PubMed]
- Zenapax [package insert]. Nutley, NJ: Roche Pharmaceuticals, 2005.
- Simulect [package insert]. East Hanover, NJ: Novartis Pharmaceutical Corporation, 2005.
- Thymoglobulin [package insert]. Cambridge, MA: Genzyme, 2008.
- ATGAM [package insert]. New York, NY: Pfizer Pharmacia and Upjohn Company, 2005.
- Palmer SM, Miralles AP, Lawrence CM, et al. Rabbit antithymocyte globulin decreases acute rejection after lung transplantation. Chest 1999;116:127-33. [PubMed]
- Garrity ER, Villanueva J, Bhorade SM, et al. Low rate of acute lung allograft rejection after the use of daclizumab, an interleukin 2 receptor antibody. Transplantation 2001;71:773-7. [PubMed]
- Borro JM, De la Torre M, Miguelez C, et al. Comparative study of basiliximab treatment in lung transplantation. Transplant Proc 2005;37:3996-8. [PubMed]
- Hachem RR, Chakinala MM, Yusen RD, et al. A comparison of basiliximab and anti-thymocyte globulin as induction agents after lung transplantation. J Heart Lung Transplant 2005;24:1320-26. [PubMed]
- Burton CM, Andersen CB, Jensen AS, et al. The incidence of acute cellular rejection after lung transplantation: a comparative study of anti-thymocyte globulin and daclizumab. J Heart Lung Transplant 2006;25:638-47. [PubMed]
- Mullen JC, Oreopoulos A, Lien DC, et al. A randomized, controlled trial of daclizumabvs anti-thymocyte globulin induction for lung transplantation. J Heart Lung Transplant 2007;26:504-10. [PubMed]
- Ailawadi G, Smith PW, Oka T, et al. Effects of induction immunosuppression regimen on acute rejection, bronchiolitis obliterans, and survival after lung transplantation. J Thorac Cardiovasc Surg 2008;135:594-602. [PubMed]
- Hartwig MG, Snyder LD, Appel JZ, et al. Rabbit anti-thymocyte globulin induction therapy does not prolong survival after lung transplantation. J Heart Lung Transplant 2008;27:547-53. [PubMed]
- Clinckart F, Bulpa P, Jamart J, et al. Basiliximab as an alternative to antithymocyte globulin for early immunosuppression in lung transplantation. Transplant Proc 2009;41:607-9. [PubMed]
- Hachem RR, Edwards LB, Yusen RD, et al. The impact of induction on survival after lung transplantation: an analysis of the international society for heart and lung transplantation registry. Clin Transplant 2008;22:603-8. [PubMed]
- Campath [package insert]. Cambridge, MA: Genzyme, 2009.
- Heidt S, Hester J, Shankar S, et al. B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naïve B cells. Am J Transplant 2012;12:1784-92. [PubMed]
- Noris M, Casiraghi F, Todeschini M, et al. Regulatory T cells and T cell depletion: role of immunosuppressive drugs. J Am Soc Nephrol 2007;18:1007-18. [PubMed]
- van Loenhout KC, Groves SC, Galazka M, et al. Early outcomes using alemtuzumab induction in lung transplantation. Interact Cardiovasc Thorac Surg 2010;10:190-4. [PubMed]
- Shyu S, Dew MA, Pilewski JM, et al. Five-year outcomes with alemtuzumab induction after lung transplantation. J Heart Lung Transplant 2011;30:743-54. [PubMed]
- Matsuda S, Koyasu S. Mechanisms of action of cyclosporine. Immunopharmacology 2000;47:119-25. [PubMed]
- Neoral [package insert]. East Hanover, NJ: Novartis Pharmaceuticals Corporation, 2013.
- Trull A, Steel L, Sharples L, et al. Randomized, trough blood cyclosporine concentration-controlled trial to compare the pharmacodynamics of Sandimmune and Neoral in de novo lung transplant recipients. Ther Drug Monit 1999;21:17-26. [PubMed]
- Grevel J, Welsh MS, Kahan BD. Cyclosporine monitoring in renal transplantation: area under the curve monitoring is superior to trough-level monitoring. Ther Drug Monit 1989;11:246-8. [PubMed]
- Dumont RJ, Partovi N, Levy RD, et al. A limited sampling strategy for cyclosporine area under the curve monitoring in lung transplant recipients. J Heart Lung Transplant 2001;20:897-900. [PubMed]
- Jaksch P, Kocher A, Neuhauser P, et al. Monitoring C2 level predicts exposure in maintenance lung transplant patients receiving the microemulsion formulation of cyclosporine (Neoral). J Heart Lung Transplant 2005;24:1076-80. [PubMed]
- Morton JM, Aboyoun CL, Malouf MA, et al. Enhanced clinical utility of de novo cyclosporine C2 monitoring after lung transplantation. J Heart Lung Transplant 2004;23:1035-9. [PubMed]
- Prograf [package insert]. Northbrook, IL: AstellasPharma US, Inc, 2013.
- Watkins KD, Boettger RF, Hanger KM, et al. Use of sublingual tacrolimus in lung transplant recipients. J Heart Lung Transplant 2012;31:127-32. [PubMed]
- Knoop C, Thiry P, Saint-Marcoux F, et al. Tacrolimus pharmacokinetics and dose monitoring after lung transplantation for cystic fibrosis and other conditions. Am J Transplant 2005;5:1477-82. [PubMed]
- Ragette R, Kamler M, Weinreich G, et al. Tacrolimus pharmacokinetics in lung transplantation: new strategies for monitoring. J Heart Lung Transplant 2005;24:1315-9. [PubMed]
- Flechner SM, Kobashigawa J, Klintmalm G. Calcineurin inhibitor-sparing regimens in solid organ transplantation: focus on improving renal function and nephrotoxicity. Clin Transplant 2008;22:1-15. [PubMed]
- Heisel O, Heisel R, Balshaw R, et al. New onset diabetes mellitus in patients receiving calcineurin inhibitors: a systematic review and meta-analysis. Am J Transplant 2004;4:583-95. [PubMed]
- McAlister VC, Haddad E, Renouf E, et al. Cyclosporin versus tacrolimus as primary immunosuppressant after liver transplantation: a meta-analysis. Am J Transplant 2006;6:1578-85. [PubMed]
- Moore R, Hernandez D, Valantine H. Calcineurin inhibitors and post-transplant hyperlipidaemias. Drug Saf 2001;24:755-66. [PubMed]
- Penninga L, Møller CH, Gustafsson F, et al. Tacrolimus versus cyclosporine as primary immunosuppression after heart transplantation: systematic review with meta-analyses and trial sequential analyses of randomised trials. Eur J Clin Pharmacol 2010;66:1177-87. [PubMed]
- Vincenti F, Friman S, Scheuermann E, et al. Results of an international, randomized trial comparing glucose metabolism disorders and outcome with cyclosporine versus tacrolimus. Am J Transplant 2007;7:1506-14. [PubMed]
- White M, Haddad H, Leblanc MH, et al. Conversion from cyclosporine microemulsion to tacrolimus-based immunoprophylaxis improves cholesterol profile in heart transplant recipients with treated but persistent dyslipidemia: the Canadian multicentre randomized trial of tacrolimus vs cyclosporine microemulsion. J Heart Lung Transplant 2005;24:798-809. [PubMed]
- Taylor JL, Palmer SM. Critical care perspective on immunotherapy in lung transplantation. J Intensive Care Med 2006;21:327-44. [PubMed]
- Griffith BP, Bando K, Hardesty RL, et al. A prospective randomized trial of FK506 versus cyclosporine after human pulmonary transplantation. Transplantation 1994;57:848-51. [PubMed]
- Treede H, Klepetko W, Reichenspurner H, et al. Tacrolimus versus cyclosporine after lung transplantation: a prospective, open, randomized two-center trial comparing two different immunosuppressive protocols. J Heart Lung Transplant 2001;20:511-7. [PubMed]
- Zuckermann A, Reichenspurner H, Birsan T, et al. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: one-year results of a 2-center prospective randomized trial. J Thorac Cardiovasc Surg 2003;125:891-900. [PubMed]
- Hachem RR, Yusen RD, Chakinala MM, et al. A randomized controlled trial of tacrolimus versus cyclosporine after lung transplantation. J Heart Lung Transplant 2007;26:1012-8. [PubMed]
- Neurohr C, Huppmann P, Zimmermann G, et al. Tacrolimus and mycophenolate mofetil as first line immunosuppression after lung transplantation. Transpl Int 2009;22:635-43. [PubMed]
- Treede H, Glanville AR, Klepetko W, et al. Tacrolimus and cyclosporine have differential effects on the risk of development of bronchiolitis obliterans syndrome: results of a prospective, randomized international trial in lung transplantation. J Heart Lung Transplant 2012;31:797-804. [PubMed]
- Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: Mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol 2005;56:23-46. [PubMed]
- Holt DW. Monitoring mycophenolic acid. Ann Clin Biochem 2002;39:173-83. [PubMed]
- Hale MD, Nicholls AJ, Bullingham RE, et al. The pharmacokinetic-pharmacodynamic relationship for mycophenolate mofetil in renal transplantation. Clin Pharmacol Ther 1998;64:672-83. [PubMed]
- Knoop C, Haverich A, Fischer S. Immunosuppressive therapy after human lung transplantation. Eur Respir J 2004;23:159-71. [PubMed]
- Ting LS, Partovi N, Levy RD, et al. Limited sampling strategy for predicting area under the concentration-time curve of mycophenolic acid in adult lung transplant recipients. Pharmacotherapy 2006;26:1232-40. [PubMed]
- Whyte RI, Rossi SJ, Mulligan MS, et al. Mycophenolate mofetil for obliterative bronchiolitis syndrome after lung transplantation. Ann Thorac Surg 1997;64:945-8. [PubMed]
- Palmer SM, Baz MA, Sanders L, et al. Results of a randomized, prospective, multicenter trial of mycophenolate mofetil versus azathioprine in the prevention of acute lung allograft rejection. Transplantation 2001;71:1772-6. [PubMed]
- McNeil K, Glanville AR, Wahlers T, et al. Comparison of mycophenolate mofetil and azathioprine for prevention of bronchiolitis obliterans syndrome in de novo lung transplant recipients. Transplantation 2006;81:998-1003. [PubMed]
- Roman A, Ussetti P, Zurbano F, et al. A retrospective 12-month study of conversion to everolimus in lung transplant recipients. Transplant Proc 2011;43:2693-8. [PubMed]
- Cahill BC, Somerville KT, Crompton JA, et al. Early experience with sirolimus in lung transplant recipients with chronic allograft rejection. J Heart Lung Transplant 2003;22:169-76. [PubMed]
- Parada MT, Alba A, Sepúlveda C, et al. Long-term use of everolimus in lung transplant patients. Transplant Proc 2011;43:2313-5. [PubMed]
- Shitrit D, Rahamimov R, Gidon S, et al. Use of sirolimus and low-dose calcineurin inhibitor in lung transplant recipients with renal impairment: results of a controlled pilot study. Kidney Int 2005;67:1471-5. [PubMed]
- Gullestad L, Iversen M, Mortensen SA, et al. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation 2010;89:864-72. [PubMed]
- Azzola A, Havryk A, Chhajed P, et al. Everolimus and mycophenolate mofetil are potent inhibitors of fibroblast proliferation after lung transplantation. Transplantation 2004;77:275-80. [PubMed]
- Hernández RL, Gil PU, Gallo CG, et al. Rapamycin in lung transplantation. Transplant Proc 2005;37:3999-4000. [PubMed]
- Villanueva J, Boukhamseen A, Bhorade SM. Successful use in lung transplantation of an immunosuppressive regimen aimed at reducing target blood levels of sirolimus and tacrolimus. J Heart Lung Transplant 2005;24:421-5. [PubMed]
- Groetzner J, Kur F, Spelsberg F, et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. J Heart Lung Transplant 2004;23:632-8. [PubMed]
- King-Biggs MB, Dunitz JM, Park SJ, et al. Airway anastomotic dehiscence associated with use of sirolimus immediately after lung transplantation. Transplantation 2003;75:1437-43. [PubMed]
- Snell GI, Valentine VG, Vitulo P, et al. Everolimus versus azathioprine in maintenance lung transplant recipients: an international, randomized, double-blind clinical trial. Am J Transplant 2006;6:169-77. [PubMed]
- Sacher VY, Fertel D, Srivastava K, et al. Effects of prophylactic use of sirolimus on bronchiolitis obliterans syndrome development in lung transplant recipients. Ann Thorac Surg 2014;97:268-74. [PubMed]
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol 2006;148:245-54. [PubMed]
- Borro JM, Solé A, De la Torre M, et al. Steroid withdrawal in lung transplant recipients. Transplant Proc 2005;37:3991-3. [PubMed]
- Shitrit D, Bendayan D, Sulkes J, et al. Successful steroid withdrawal in lung transplant recipients: result of a pilot study. Respir Med 2005;99:596-601. [PubMed]
- Stewart S, Fishbein MC, Snell GI, et al. Revision of the 1996 working formulation for the standardization of nomenclature in the diagnosis of lung rejection. J Heart Lung Transplant 2007;26:1229-42. [PubMed]
- Berry G, Burke M, Andersen C, et al. Pathology of pulmonary antibody-mediated rejection: 2012 update from the Pathology Council of the ISHLT. J Heart Lung Transplant 2013;32:14-21. [PubMed]
- Glanville AR. Antibody-mediated rejection in lung transplantation: myth or reality? J Heart Lung Transplant 2010;29:395-400. [PubMed]
- Appel JZ 3rd, Hartwig MG, Davis RD, et al. Utility of peritransplant and rescue intravenous immunoglobulin and extracorporeal immunoadsorption in lung transplant recipients sensitized to HLA antigens. Hum Immunol 2005;66:378-86. [PubMed]
- Hachem RR, Yusen RD, Meyers BF, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant 2010;29:973-80. [PubMed]
- Astor TL, Weill D, Cool C, et al. Pulmonary capillaritis in lung transplant recipients: treatment and effect on allograft function. J Heart Lung Transplant 2005;24:2091-7. [PubMed]
- Baum C, Reichenspurner H, Deuse T. Bortezomib rescue therapy in a patient with recurrent antibody-mediated rejection after lung transplantation. J Heart Lung Transplant 2013;32:1270-1. [PubMed]
- Neumann J, Tarrasconi H, Bortolotto A, et al. Acute humoral rejection in a lung recipient: reversion with bortezomib. Transplantation 2010;89:125-6. [PubMed]
- Hachem R. Antibody-Mediated Lung Transplant Rejection. Curr Respir Care Rep 2012;1:157-61. [PubMed]
- Bittner HB, Dunitz J, Hertz M, et al. Hyperacute rejection in single lung transplantation--case report of successful management by means of plasmapheresis and antithymocyte globulin treatment. Transplantation 2001;71:649-51. [PubMed]
- Dawson KL, Parulekar A, Seethamraju H. Treatment of hyperacute antibody-mediated lung allograft rejection with eculizumab. J Heart Lung Transplant 2012;31:1325-6. [PubMed]
- Iacono AT, Keenan RJ, Duncan SR, et al. Aerosolized cyclosporine in lung recipients with refractory chronic rejection. Am J Respir Crit Care Med 1996;153:1451-5. [PubMed]
- Iacono AT, Smaldone GC, Keenan RJ, et al. Dose-related reversal of acute lung rejection by aerosolized cyclosporine. Am J Respir Crit Care Med 1997;155:1690-8. [PubMed]
- Iacono AT, Corcoran TE, Griffith BP, et al. Aerosol cyclosporin therapy in lung transplant recipients with bronchiolitis obliterans. Eur Respir J 2004;23:384-90. [PubMed]
- Iacono AT, Johnson BA, Grgurich WF, et al. A randomized trial of inhaled cyclosporine in lung-transplant recipients. N Engl J Med. 2006;354:141-50. [PubMed]
- Watts AB, Cline AM, Saad AR, et al. Characterization and pharmacokinetic analysis of tacrolimus dispersion for nebulization in a lung transplanted rodent model. Int J Pharm 2010;384:46-52. [PubMed]
- Watts AB, Peters JI, Talbert RL, et al. Preclinical evaluation of tacrolimus colloidal dispersion for inhalation. Eur J Pharm Biopharm 2011;77:207-15. [PubMed]
- Bayer J, Das NA, Baisden CE, et al. Effect of inhaled tacrolimus on ischemia reperfusion injury in rat lung transplant model. J Thorac Cardiovasc Surg 2013;146:1213-9; discussion 1219. [PubMed]
- Hayes D Jr, Zwischenberger JB, Mansour HM. Aerosolized tacrolimus: a case report in a lung transplant recipient. Transplant Proc 2010;42:3876-9. [PubMed]
- Vos R, Vanaudenaerde BM, Verleden SE, et al. Anti-inflammatory and immunomodulatory properties of azithromycin involved in treatment and prevention of chronic lung allograft rejection. Transplantation 2012;94:101-9. [PubMed]
- Jaffé A, Francis J, Rosenthal M, et al. Long-term azithromycin may improve lung function in children with cystic fibrosis. Lancet 1998;351:420. [PubMed]
- Wolter J, Seeney S, Bell S, et al. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomised trial. Thorax 2002;57:212-6. [PubMed]
- Gerhardt SG, McDyer JF, Girgis RE, et al. Maintenance azithromycin therapy for bronchiolitis obliterans syndrome: results of a pilot study. Am J Respir Crit Care Med 2003;168:121-5. [PubMed]
- Verleden GM, Dupont LJ. Azithromycin therapy for patients with bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2004;77:1465-7. [PubMed]
- Yates B, Murphy DM, Forrest IA, et al. Azithromycin reverses airflow obstruction in established bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2005;172:772-5. [PubMed]
- Gottlieb J, Szangolies J, Koehnlein T, et al. Long-term azithromycin for bronchiolitis obliterans syndrome after lung transplantation. Transplantation 2008;85:36-41. [PubMed]
- Porhownik NR, Batobara W, Kepron W, et al. Effect of maintenance azithromycin on established bronchiolitis obliterans syndrome in lung transplant patients. Can Respir J 2008;15:199-202. [PubMed]
- Vos R, Vanaudenaerde BM, Ottevaere A, et al. Long-term azithromycin therapy for bronchiolitis obliterans syndrome: divide and conquer? J Heart Lung Transplant 2010;29:1358-68. [PubMed]
- Verleden GM, Vanaudenaerde BM, Dupont LJ, et al. Azithromycin reduces airway neutrophilia and interleukin-8 in patients with bronchiolitis obliterans syndrome. Am J Respir Crit Care Med 2006;174:566-70. [PubMed]
- Federica M, Nadia S, Monica M, et al. Clinical and immunological evaluation of 12-month azithromycin therapy in chronic lung allograft rejection. Clin Transplant 2011;25:E381-9. [PubMed]
- Jain R, Hachem RR, Morrell MR, et al. Azithromycin is associated with increased survival in lung transplant recipients with bronchiolitis obliterans syndrome. J Heart Lung Transplant 2010;29:531-7. [PubMed]
- Vos R, Vanaudenaerde BM, Verleden SE, et al. A randomised controlled trial of azithromycin to prevent chronic rejection after lung transplantation. Eur Respir J 2011;37:164-72. [PubMed]
- Morrell MR, Despotis GJ, Lublin DM, et al. The efficacy of photopheresis for bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant 2010;29:424-31. [PubMed]
- Jaksch P, Scheed A, Keplinger M, et al. A prospective interventional study on the use of extracorporeal photopheresis in patients with bronchiolitis obliterans syndrome after lung transplantation. J Heart Lung Transplant 2012;31:950-7. [PubMed]
- Greer M, Dierich M, De Wall C, et al. Phenotyping established chronic lung allograft dysfunction predicts extracorporeal photopheresis response in lung transplant patients. Am J Transplant 2013;13:911-8. [PubMed]
- Ropponen JO, Syrjälä SO, Hollmén M, et al. Effect of simvastatin on development of obliterative airway disease: an experimental study. J Heart Lung Transplant 2012;31:194-203. [PubMed]
- Mausner-Fainberg K, Luboshits G, Mor A, et al. The effect of HMG-CoA reductase inhibitors on naturally occurring CD4+CD25+ T cells. Atherosclerosis 2008;197:829-39. [PubMed]
- Kwak B, Mulhaupt F, Myit S, et al. Statins as a newly recognized type of immunomodulator. Nat Med 2000;6:1399-402. [PubMed]
- Watts KL, Sampson EM, Schultz GS, et al. Simvastatin inhibits growth factor expression and modulates profibrogenic markers in lung fibroblasts. Am J Respir Cell Mol Biol 2005;32:290-300. [PubMed]
- Johnson BA, Iacono AT, Zeevi A, et al. Statin use is associated with improved function and survival of lung allografts. Am J Respir Crit Care Med 2003;167:1271-8. [PubMed]
- Li Y, Gottlieb J, Ma D, et al. Graft-protective effects of the HMG-CoA reductase inhibitor pravastatin after lung transplantation--a propensity score analysis with 23 years of follow-up. Transplantation 2011;92:486-92. [PubMed]
- Margolin SB. eds. Investigation of new drug: pirfenidone. Dallas, TX: Marnac, Inc, 2002.
- Dosanjh AK, Wan B, Throndset W, et al. Pirfenidone: a novel antifibrotic agent with implications for the treatment of obliterative bronchiolitis. Transplant Proc 1998;30:1910-1. [PubMed]
- Zhou H, Latham CW, Zander DS, et al. Pirfenidone inhibits obliterative airway disease in mouse tracheal allografts. J Heart Lung Transplant 2005;24:1577-85. [PubMed]
- Visner GA, Liu F, Bizargity P, et al. Pirfenidone inhibits T-cell activation, proliferation, cytokine and chemokine production, and host alloresponses. Transplantation 2009;88:330-8. [PubMed]
- Bizargity P, Liu K, Wang L, et al. Inhibitory effects of pirfenidone on dendritic cells and lung allograft rejection. Transplantation 2012;94:114-22. [PubMed]
- Ihle F, von Wulffen W, Neurohr C. Pirfenidone: a potential therapy for progressive lung allograft dysfunction? J Heart Lung Transplant 2013;32:574-5. [PubMed]
- Vos R, Verleden SE, Ruttens D, et al. Pirfenidone: a potential new therapy for restrictive allograft syndrome? Am J Transplant 2013;13:3035-40. [PubMed]