
A novel risk assessment model for venous thromboembolism after major thoracic surgery: a Chinese single-center study
Introduction
Venous thromboembolism (VTE) is an insidious disease with significant morbidity and mortality. VTE is often undiagnosed or misdiagnosed because it lacks specific symptoms. VTE includes deep vein thrombosis (DVT) and pulmonary embolism (PE), which share common predisposing factors and represent similar entities at different sites and stages. DVT and PE are important causes of unexpected death in hospitals. Therefore, VTE has been extensively studied worldwide in recent years (1-5).
It was shown that approximately 500,000 VTE events occur in the USA per year, almost half of which are related to recent or current inpatient care experiences. In the inpatients of Mayo Clinic from 2005 to 2010, the percentage of patients who received VTE prophylaxis or unnecessary VTE prophylaxis after evaluation increased from 40% to 90% (6). In thoracic surgery clinics, VTE is an insidious disease with nonspecific symptoms that are easily overlooked by inexperienced eyes.
There are a variety of risk assessment models (RAMs) for VTE in Western countries, including the Caprini RAM (7) (based on the modified scoring system used at Boston Medical Center; applicable for medical and surgical inpatients), Rogers RAM (8) (applicable for surgical inpatients except orthopedics), Padua RAM (9) (applicable for medical inpatients), and Khorana RAM (10) (applicable for oncological patients).
To ensure that all hospitalized patients are being assessed and receive adequate thromboprophylaxis, individual VTE risk assessment plays an important role. These models are also widely employed in Asian populations because the acquired risk factors for VTE are similar in both Asian and Caucasians (11). However, criteria endorsed by guidelines from Western countries cannot be recommended directly for Chinese populations until they are definitely validated in multi-center, prospective validation studies in a large cohort. In this study, we applied several models to determine the possible risk factors for VTE in the Chinese thoracic surgical population. Subsequently, we tried to define risk stratification scores and develop a simply VTE RAM applicable to Chinese thoracic surgical patients. Then we established an individual prophylaxis strategy and reasonable VTE prophylaxis protocol.
Methods
Patients
This retrospective study was approved by the institutional research ethics committee of the Beijing Chao-Yang Hospital. Patients who underwent thoracic surgery at Beijing Chao-Yang Hospital were enrolled in this study (n=533). From July 2016 to December 2017, 837 patients had undergone thoracic surgery at the Department of Thoracic Surgery of Beijing Chao-Yang Hospital. Among these patients, 120 patients who missed VTE-related image or blood test; 168 patients who received perioperative prophylaxis; 7 patients who had been confirmed with VTE before surgery; 7 patients who died because of non-VTE-related postoperative complications, and 2 patients who had superficial vein thrombosis (SVT) after operation were excluded from the study. General and operation information for patients and laboratory blood test results was collected.
Lower limbs Doppler ultrasonography was performed before and after surgery for deep venous thrombosis (DVT) confirmation. Patients with new postoperative DVT, typical symptoms of PE, or high Caprini score (≥9) underwent further computer tomography pulmonary angiography (CTPA) examination for PE. The VTE incidence was evaluated before discharge.
Caprini, Rogers, Padua, and Khorana RAM were applied for all of the patients. A novel RAM of VTE, which we called Chao-Yang VTE RAM, was developed according to the logistic regression analysis. We used the receiver operating characteristic (ROC) curve to discriminate patients with and without VTE. In this study, areas under the ROC curve of 0.5–0.7, 0.7–0.9, and >0.9 were considered as low, moderate, and high discrimination, respectively.
Patients were divided into three categories according to their score: low-risk group (0–4 points), moderate-risk group (5–8 points), and high-risk group (≥9 points).
Inclusion criteria
The medical records of patients who underwent thoracic surgery were reviewed. The follow-up period ended upon patients’ discharge from the hospital.
Exclusion criteria
Patients with missing information, especially those who did not undergo VTE-related imaging or blood test before or after surgery; patients who had received any perioperative prophylaxis; patients who had been confirmed with VTE before surgery; patients who died because of non-VTE-related postoperative complications, and patients who had superficial vein thrombosis (SVT) after operation.
Statistical analysis
SPSS 23.0 and SigmaPlot 12.0 software for Windows were used for statistical analysis. Continuous variables were expressed as the mean ± standard deviation (SD) or medians and were compared by either student’s t test or the Mann-Whitney U test. Categorical variables were compared by χ2 test or Fisher’s exact test. Single-factor analysis was used to define risk factors associated with VTE. We performed regression analysis and set the P value for selection as an independent variable at 0.05. The null hypothesis was rejected for P<0.05.
Results
From the Department of Thoracic Surgery, Beijing Chao-Yang Hospital, Capital Medical University, 533 patients were enrolled in this study from July 2016 to December 2017, including 285 male patients and 248 female patients. The mean age of these patients was 52.6 years. The overall incidence of VTE after thoracic surgery was 8.4% (45 of 533). Among 45 VTE patients, 86.7% were DVT and 13.3% were DVT + PE. Specifically, the incidence of VTE in patients who received lung surgery was 7.7% (34 of 444), while the incidence of VTE after esophagus surgery, mediastinal surgery, and other surgery was 20.0% (6 of 30), 5.0% (2 of 40), and 15.8% (3 of 19), respectively.
There was no difference in terms of sex between patients with and without VTE. However, the mean surgery age of patients who developed VTE was significantly greater than of those without VTE (64.29 versus 51.52 years, P=0.000). Furthermore, the BMI in VTE patients was higher than in those without VTE (24.78 versus 23.24 kg/m2, P=0.013). We also found that operative time and intraoperative bleeding of VTE patients were higher than those of non-VTE patients (197.11 versus 143.63 min, P=0.000 and 257.56 versus 152.99 mL, P=0.000). Postoperative VTE incidence was correlated with an increased American Society of Anesthesiologists (ASA) score. The VTE incidence in the 1 and 2–5 ASA scores was 2.5% and 9.5%, respectively. The high-ASA patients had a significantly higher incidence of VTE than low-ASA patients (P=0.036). The VTE incidence in patients with cancer diseases after surgery was 12.2% (36 of 296), which was higher than those with benign diseases 3.8% (9 of 237), P=0.001. Furthermore, VTE incidence in patients who received open surgery was higher than in those who received video-assisted thoracic surgery (VATS) (15.1% versus 6.5%, P=0.003) (Table 1).
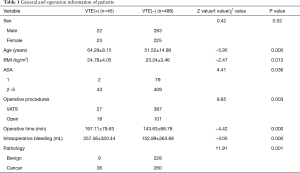
Full table
We found that the levels of pre-operative D-Dimer in patients who developed VTE after thoracic surgery were much higher than in patients without VTE: 0.69 versus 0.41 mg/L FEU, P=0.000; but red blood cell (RBC) counts in patients who developed VTE after thoracic surgery were much lower than in patients without VTE: 4.32 versus 4.54×1012/L, P=0.028. However, there were no differences in terms of the levels of white blood cell (WBC) (6.29×109/L versus 6.42×109/L, P=0.60), platelet (PLT) (259.29×109/L versus 241.61×109/L, P=0.24), in patients with and without VTE (Table 2).
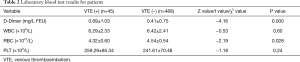
Full table
Following regression analysis, we found that age ≥60 (OR 4.51, 95% CI: 2.09–9.71, P=0.000) was independent risk factor for VTE. And ASA ≥2 (OR 1.34, 95% CI: 0.29–6.25, P=0.71); pathology (Benign =0 or Cancer =2) (OR 1.80, 95% CI: 0.79–4.11, P=0.16); operative procedures (VATS =0 or Open =2) (OR 1.51, 95% CI: 0.72–3.19, P=0.28); operative time (>180 min) (OR 1.82, 95% CI: 0.85–3.89, P=0.13); intraoperative bleeding (>200 mL) (OR 1.54, 95% CI: 0.64–3.71, P=0.34); D-Dimer >0.55 (mg/L FEU) (OR 1.69, 95% CI: 0.77–3.72, P=0.19); RBC <4.0×1012/L (OR 1.71, 95% CI: 0.78–3.75, P=0.18); and BMI (≥30 kg/m2) (OR 2.21, 95% CI: 0.59–8.30, P=0.24) were not independent risk factors for VTE (Table 3).
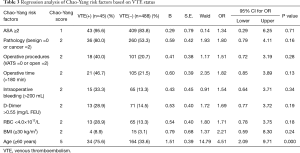
Full table
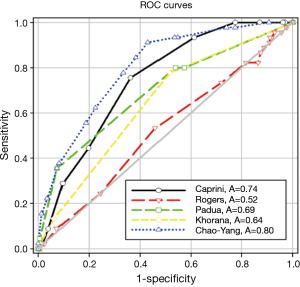
A novel RAM of VTE was developed according to the regression analysis and based on clinical experience. We called this new RAM the Chao-Yang VTE RAM (Table 3). The areas under the ROC curve of Caprini, Rogers, Padua, Khorana, and Chao-Yang models were 0.74 (P<0.0001), 0.52 (P=0.62), 0.69 (P<0.0001), 0.64 (P=0.0017), and 0.80 (P<0.0001), respectively (Figure 1). In the Caprini model, when the Youden index was >5.5, the sensitivity and specificity were 0.76 and 0.64, respectively. Similarly, in the Rogers model, when the Youden index was >14.5, the sensitivity and specificity were 0.53 and 0.54, respectively. For the Padua model, when the Youden index was >3.5, the sensitivity was 0.36, and the specificity was 0.93. For the Khorana model, when the Youden index was >0.5, the sensitivity was 0.78, and the specificity was 0.48. Finally, with the Chao-Yang model, when the Youden index was >5.5, the sensitivity and specificity for the prediction of VTE were 0.91 and 0.57, respectively (Table 4).
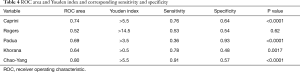
Full table
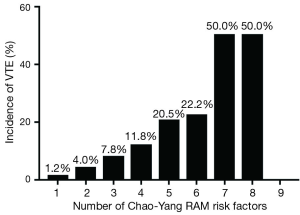
The VTE incidence in the low-, moderate-, and high-risk groups predicted with Chao-Yang scores was 1.3% (3 of 230), 8.4% (14 of 166), and 20.4% (28 of 137); these were 1.6% (3 of 192), 11.9% (38 of 318), and 17.4% (4 of 23), respectively, when using Caprini criteria. The high-risk patients’ group had a significantly higher incidence than the low- and moderate-risk groups (P=0.000) (Table 5). Additionally, as the number of risk factors increased, the incidence of VTE increased from 1.2% to 50.0% (Figure 2).

Full table
Discussion
In the last decade, VTE has gained increasing interest worldwide. A number of RAMs for VTE have been developed by professional societies and institutions. VTE is common in the thoracic surgical population (12,13) because thoracic surgery typically involves many large blood vessels and is highly invasive. In addition, each existing model includes many different risk factors, which require that the reviewer is extremely familiar with the patient’s history and laboratory test results and that the reviewer dynamically scores in a timely fashion when risk factors change. Furthermore, there are many problems with these models if they are directly employed in the Chinese population because the consensus has been achieved based on Western populations (14). However, no VTE RAM specific for Chinese thoracic population is available, thus leading us to the retrospective study.
Overall, 8.4% patients after thoracic surgery developed VTE, which was higher than previously thought in the thoracic surgical population (7). The main type of VTE was DVT, and there was no pure PE; all PE was caused by DVT. Blood clots first formed in the lower extremities and then migrated to the pulmonary vessels. So lower-extremity ultrasonography for all patients after thoracic surgery was an effective method to detect VTE after surgery (15,16). In subgroup analysis, 7.7% patients after lung surgery, similar with previous study (17), and 20.0% patients after esophagus surgery developed VTE. The incidence of VTE after esophageal surgery was much higher than the incidence of VTE after lung surgery. Compared with lung surgery, esophagus surgery had a longer operative time and longer stay in bedtime after operation; these were risk factors to developed VTE (18). This study still has bias; in our study, 83.3% was lung surgery, and only 5.6% was esophagus surgery, because the background and intervention between the patients who underwent lung surgery and other surgery was different. In our later work, we need to increase the data of other types of surgery.
Based on regression analyses and clinical experience, we established the Chao-Yang VTE RAM. This model considered the following: (I) patient features such as age and BMI; (II) clinical test results such as RBC counts and D-Dimer levels; (III) intraoperative features such as ASA scores, operative time, intraoperative bleeding, and operative procedure (open versus VATS); and (IV) postoperative features, such as histopathology analysis. After a single-factor analysis, we included independent variables in the logistic regression model, which had univariate significant differences and independent variable that was clinically considered to be closely related to the dependent variable. We found that age ≥60 was an independent risk factor for VTE. In addition, ASA ≥2, cancer, and open surgery, operative time >180 min, intraoperative bleeding >200 mL, D-Dimer >0.55 (mg/L FEU), RBC <4.0×1012/L, and BMI (≥30 kg/m2) were risk factors for VTE, but there were no differences in PT APTT INR in our previous study (19). According to the OR value of regression analysis, we set the score to our Chao-Yang RAM.
Comparing Chao-Yang RAM with Caprini, Rogers, Padua, and Khorana RAM, Chao-Yang RAM, and Caprini RAM had a high area under the ROC curve. Chao-Yang RAM and Caprini RAM were 0.80 and 0.74, respectively (P<0.0001). They provided a moderate discrimination of patients with and without VTE. Furthermore, when we set the Youden index as the cutoff value, Chao-Yang RAM and Caprini RAM both did not have a very high specificity. After we adjusted the model with risk stratification, we determined 0–4 points, 5–8 points, and ≥9 points to represent low-risk, moderate-risk, and high-risk groups, respectively (7,20). The Chao-Yang model and Caprini model were effective in predicting VTE events in the low-risk (1.3% versus 1.6%), moderate-risk (8.4% versus 11.9%), and high-risk (20.4% versus 17.4%) groups, especially in the high-risk group correlated well in identifying patients at high risk for VTE. In our study, because we need to get a real-world rate of VTE after thoracic surgery, we excluded 168 patients receiving perioperative prophylaxis. However, in the real world, the patients after thoracic surgery (especially in high-risk patients) often take some prophylaxis, so our result of high-risk groups rate of VTE was lower than the real world, but the results were still high, suggesting that we should pay more attention to patients who are at high risk.
Notably, the Chao-Yang model was more accurate in predicting VTE events in the high-risk group, which requires clinicians to be vigilant for VTE in individuals scoring ≥9 points. Compared to Caprini RAM, Chao-Yang RAM was based on surgery clinical practice and had fewer risk factors, so it was easier to use.
Additionally, as the number of risk factors increased, the incidence of VTE increased from 1.2% to 50.0%. Some studies (21) have suggested that stratification of patients with the VTE model would facilitate management of different patients with specific VTE prophylaxis strategies [e.g., encourage early exercise or mechanical prophylaxis for patients with low-risk, intermittent pneumatic compression (IPC), or low molecular weight heparin (LMWH) prophylaxis for patients with moderate risk (22), or concomitant IPC and LMWH prophylaxis for patients with high risk (23)], thus preventing under- and over-prophylaxis. However, another study (6) suggested that hospitalization-related VTE attack rates did not change significantly, despite the initiation of comprehensive VTE evaluation and prophylaxis for those patients. The duration of hospital stays and inpatient VTE prophylaxis duration in that study were 3 days and 70 h, respectively, and 75% VTE events occurred after discharge (median time 19.5 days).
Moreover, each model is directed by different guiding strategies, which are grossly classified into collective strategy [i.e., VTE prophylaxis is applied to patients with the same condition, which is seen in the American College of Chest Physicians (ACCP) guideline recommendations for VTE risk assessment for orthopedic patients (24)] or individual strategy [i.e., VTE prophylaxis is recommended based on risk assessment for every single patient, which is seen in the ACCP guideline recommendations for VTE risk assessment for emergency general surgical patients (25)]. Individual strategies are increasingly recommended for clinical practice. Further studies should focus on ambulatory patients and inpatients with a high risk of developing VTE, establishing prevention strategies on case-by-case bases and designing proper VTE prophylaxis protocols.
Ideally, each population should be served by specific VTE assessment and recommended prophylaxis. However, no RAM has been recently validated in Asians. To develop a preferable and validated Asian model through collaborative efforts, further studies are required (11). Currently, the criteria for VTE risk stratification are highly sensitive but poorly specific, resulting in almost all oncological patients being rated as high-risk (26).
In summary, we developed a Chao-Yang VTE RAM and compared it with other widely accepted models in a Chinese thoracic surgery cohort. This model had adequate power in identifying patients with different risks for VTE events and was somewhat better than the Caprini model. Therefore, the Chao-Yang model may be applicable in predicting the occurrence of VTE in Chinese patients receiving thoracic surgery. However, this study was carried out in only one large center, which limits its usefulness and dissemination. Hopefully, this model will be further validated in a large, multi-center, retrospective validation study before providing benefits for Chinese patients.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Research Ethics Committee of Beijing Chao-Yang Hospital, Capital Medical University (ID: 2017-Ke-1).
References
- Stone J, Hangge P, Albadawi H, et al. Deep vein thrombosis: pathogenesis, diagnosis, and medical management. Cardiovasc Diagn Ther 2017;7:S276-S284. [Crossref] [PubMed]
- Silverstein MD, Heit JA, Mohr DN, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med 1998;158:585-93. [Crossref] [PubMed]
- Heit JA, Melton LJ 3rd, Lohse CM, et al. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin Proc 2001;76:1102-10. [Crossref] [PubMed]
- Dentali F, Malato A, Ageno W, et al. Incidence of venous thromboembolism in patients undergoing thoracotomy for lung cancer. J Thorac Cardiovasc Surg 2008;135:705-6. [Crossref] [PubMed]
- Heit JA, Silverstein MD, Mohr DN, et al. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case-control study. Arch Intern Med 2000;160:809-15. [Crossref] [PubMed]
- Heit JA, Crusan DJ, Ashrani AA, et al. Effect of a near-universal hospitalization-based prophylaxis regimen on annual number of venous thromboembolism events in the US. Blood 2017;130:109-14. [Crossref] [PubMed]
- Hachey KJ, Hewes PD, Porter LP, et al. Caprini venous thromboembolism risk assessment permits selection for postdischarge prophylactic anticoagulation in patients with resectable lung cancer. J Thorac Cardiovasc Surg 2016;151:37-44 e1.
- Rogers SO Jr, Kilaru RK, Hosokawa P, et al. Multivariable predictors of postoperative venous thromboembolic events after general and vascular surgery: results from the patient safety in surgery study. J Am Coll Surg 2007;204:1211-21. [Crossref] [PubMed]
- Barbar S, Noventa F, Rossetto V, et al. A risk assessment model for the identification of hospitalized medical patients at risk for venous thromboembolism: the Padua Prediction Score. J Thromb Haemost 2010;8:2450-7. [Crossref] [PubMed]
- Khorana AA, Dalal M, Lin J, et al. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer 2013;119:648-55. [Crossref] [PubMed]
- Liew NC, Alemany GV, Angchaisuksiri P, et al. Asian venous thromboembolism guidelines: updated recommendations for the prevention of venous thromboembolism. Int Angiol 2017;36:1-20. [PubMed]
- Hewes PD, Hachey KJ, Zhang XW, et al. Evaluation of the Caprini Model for Venothromboembolism in Esophagectomy Patients. Ann Thorac Surg 2015;100:2072-8. [Crossref] [PubMed]
- Christensen TD, Vad H, Pedersen S, et al. Venous thromboembolism in patients undergoing operations for lung cancer: a systematic review. Ann Thorac Surg 2014;97:394-400. [Crossref] [PubMed]
- Shargall Y, Litle VR. European perspectives in Thoracic Surgery, the ESTS venous thromboembolism (VTE) working group. J Thorac Dis 2018;10:S963-S968. [Crossref] [PubMed]
- Geerts WH, Bergqvist D, Pineo GF, et al. Prevention of venous thromboembolism: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest 2008;133:381S-453S.
- Lyman GH, Khorana AA, Falanga A, et al. American Society of Clinical Oncology guideline: recommendations for venous thromboembolism prophylaxis and treatment in patients with cancer. J Clin Oncol 2007;25:5490-505. [Crossref] [PubMed]
- Song C, Shargall Y, Li H, et al. Prevalence of venous thromboembolism after lung surgery in China: a single-centre, prospective cohort study involving patients undergoing lung resections without perioperative venous thromboembolism prophylaxis. Eur J Cardiothorac Surg 2018. [Epub ahead of print]. [PubMed]
- Sebastian AS, Currier BL, Kakar S, et al. Risk Factors for Venous Thromboembolism following Thoracolumbar Surgery: Analysis of 43,777 Patients from the American College of Surgeons National Surgical Quality Improvement Program 2005 to 2012. Global Spine J 2016;6:738-43. [Crossref] [PubMed]
- Tian B, Song C, Li H, et al. The significance of perioperative coagulation and fibrinolysis related parameters after lung surgery for predicting venous thromboembolism: a prospective, single center study. J Thorac Dis 2018;10:2223-30. [Crossref] [PubMed]
- Hachey KJ, Sterbling H, Choi DS, et al. Prevention of Postoperative Venous Thromboembolism in Thoracic Surgical Patients: Implementation and Evaluation of a Caprini Risk Assessment Protocol. J Am Coll Surg 2016;222:1019-27. [Crossref] [PubMed]
- Li H, Jiang G, Bolukbas S, et al. The Society for Translational Medicine: the assessment and prevention of venous thromboembolism after lung cancer surgery. J Thorac Dis 2018;10:3039-53. [Crossref] [PubMed]
- Monreal M. Long-term treatment of venous thromboembolism with low-molecular-weight heparin. Curr Opin Pulm Med 2000;6:326-9. [Crossref] [PubMed]
- Afshari A, Fenger-Eriksen C, Monreal M, et al. European guidelines on perioperative venous thromboembolism prophylaxis: Mechanical prophylaxis. Eur J Anaesthesiol 2018;35:112-5. [PubMed]
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e278S-e325S.
- Gould MK, Garcia DA, Wren SM, et al. Prevention of VTE in nonorthopedic surgical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012;141:e227S-e277S.
- Zhou HX, Peng LQ, Yan Y, et al. Validation of the Caprini risk assessment model in Chinese hospitalized patients with venous thromboembolism. Thromb Res 2012;130:735-40. [Crossref] [PubMed]


