Robot-assisted esophagogastric reconstruction in minimally invasive Ivor Lewis esophagectomy
Introduction
During the last two decades, minimally invasive esophagectomy (MIE) via thoracoscopy and laparoscopy has been implemented extensively to reduce perioperative morbidity and mortality (1-4). Most surgeons prefer the McKeown approach to obviate the creation of intrathoracic anastomosis with non-ergonomic instruments using traditional endoscopy (5). However, as the patients with the esophagogastric junction cancer are more eligible for Ivor Lewis esophagectomy, it seems necessary to simplify the endoscopic anastomosis through the application of improved techniques and advanced equipment.
In contrast to classic endoscopy, the robotic surgical system can provide high-resolution 3D images and multiarticulate instruments with tremor filtration, which can facilitate sophisticated surgical manipulation such as stitching and knotting (6,7). Therefore, performing robotic Ivor Levis esophagectomy is potentially more practical as it can overcome the difficulties of the endoscopic anastomosis. Considering this advantage, in December 2016, we began performing Ivor Lewis MIE with a surgical robot instead of classic endoscopy (8). At this time, more than 70 cases of McKeown robot-assisted minimally invasive esophagectomy (RAMIE) had been completed before we began the Ivor Lewis RAMIE (9).
The purpose of this article is to introduce our initial experience of Ivor Lewis RAMIE, a technique which focuses on the intracorporeal reconstruction of the alimentary tract using a surgical robot. The perioperative data were analyzed to explore the safety and feasibility of this approach.
Methods
Study population
The study population was a consecutive series of patients undergoing Ivor Lewis RAMIE with curative intent for esophageal cancer in the Thoracic Surgery Department at West China Hospital of Sichuan University between December 2016 and June 2018. Demographic and perioperative data were retrieved from electronic medical records, and intraoperative data were collected in real time. There was no missing data. This study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2017-239). Each patient gave consent before the operation. All patients underwent a preoperative evaluation, including history and physical examination, upper gastrointestinal endoscopy and biopsy, and thoracic and abdominal computed tomography.
Operative technique
Ivor Lewis RAMIE was performed in two stages. During the first stage (abdominal phase), the patients were placed in the supine position for gastric mobilization and construction of the tubular stomach. In the second stage (thoracic phase), the patients were repositioned in a left semi-prone position for esophageal mobilization with mediastinal lymphadenectomy and performance of the intrathoracic anastomosis.
Abdominal phase
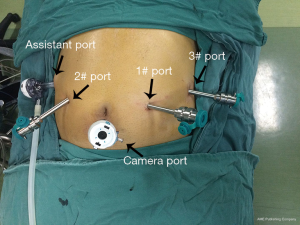
The patient was placed in the reverse Trendelenburg position, and artificial CO2 pneumoperitoneum was established to a pressure of 12 mmHg. Five ports were introduced in the upper abdomen. A12-mm port was located at the lower edge of the umbilicus for the camera, and another 12-mm port for the assistant was placed at the right anterior axillary line, about 2 cm below the costal arch. Three 8-mm ports were used for the robotic instrument arms: the arm 3 port was symmetrical with the assistant port at the left upper abdomen; the arm 1 and 2 ports were located at the right and left midclavicular line, respectively, about 2 cm above the umbilicus (Figure 1).
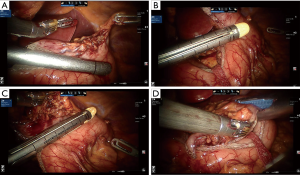
After completing gastric mobilization and abdominal lymph node dissection as previously described (10), the robot-assisted creation of the gastric tube was started at the angular notch. With the robotic instrument arms 3 and 2 grasping the lesser curve at the sites approximating the cardia and incisura, respectively, the stomach was lifted. The linear stapler (ENDOPATH® ETS 60 articulating linear cutters; Ethicon Endo-Surgery) was inserted through the assistant port to complete the first fire with the gastric antrum cut into two parts: the lesser curvature to be resected and the greater curvature to be tubular. To protect the tabularized portion of the stomach from grasping, it was encircled with a polyester tape. Position adjustment of the stomach was achieved by grasping this tape and resected lesser curvature with the robotic instruments (Figure 2).
After the tubularization of the gastric antrum was completed, robotic arm 1 was withdrawn, the 8-mm robotic cannula was replaced with a 12-mm trocar, and the stapler was introduced into the abdomen through this trocar to accommodate the orientation of the greater gastric curvature. Construction of the gastric conduit proceeded along the greater gastric curvature with a width of ~4 cm. The gastroesophageal junction was left untransected, in order to facilitate delivery of the gastric conduit into the chest. The staple line was reinforced using a 3/0 self-locking barbed suture (Stratafix™ 3-0; Ethicon).
Thoracic phase
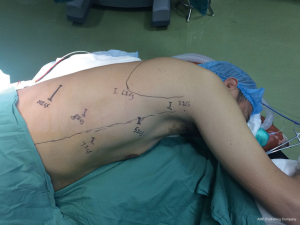
The patients were placed in the left semi-prone position with left-lung ventilation through a double-lumen endotracheal tube. Five ports were introduced for esophageal mobilization. One 12-mm port was placed below the scapular tip in the sixth intercostal space to hold the camera. Three 8-mm robotic ports were placed as follows: robotic arms 1 and 2 ports were placed at the fifth and eighth intercostal space of the posterior axillary line respectively, and robotic arm 3 port was placed at the third intercostal space of the anterior margin of the scapula. A fifth 12-mm supplementary port for assistance was placed at the seventh intercostal space of the posterior axillary line (Figure 3). The robotic cart comes over the patient’s right shoulder posteriorly.
After completion of esophageal mobilization and lymph node dissection, a 4-cm mini-thoracotomy was created at the tenth intercostal space posterior to the scapular line, and a wound protector was placed for the passage of the circular stapler and surgical specimen. With a polyester tape through this incision, the esophagus was retracted from the esophageal bed to facilitate the anastomotic procedure. A 2-cm longitudinal esophagectomy was performed below the predetermined anastomotic level with a monopolar hook cautery, through which a 25-mm EEA anvil was placed in the proximal esophagus. The anvil was secured with robotically sewn purse-string sutures (3-0 Prolene, W8977; Ethicon), and the esophagus was transected.
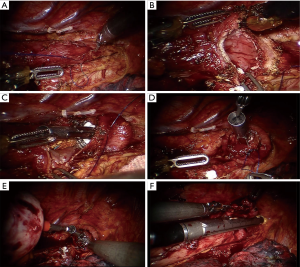
The stomach was delivered into the right hemithorax and transected at the tip of the gastric tube with the ultrasonic scalpel. After removing the separated lesser curvature and esophageal specimen, the circular stapler was introduced into the gastric tube through this incision. Under direct surveillance of the robotic 3D view, the stapler spike pierced the greater curvature and was docked with the anvil with the aid of robotic graspers. After the completion of the anastomosis, the gastrostomy was closed using a linear stapler, and the redundant gastric tip was simultaneously removed (Figure 4).
Results
Twenty-four patients (21 males and 3 females) underwent Ivor Lewis RAMIE from December 2016 to June 2018. The median age at operation was 63 years (range, 49–77 years). Other characteristics are summarized in Table 1.

Full table
The perioperative outcomes are shown in Table 2. The median estimated blood loss was 120 mL (range, 50–210 mL). The median operating time (from incision to wound closure, inclusive of docking and repositioning time) was 352.5 min (range, 259–485 min). There was no conversion to an open surgical procedure.
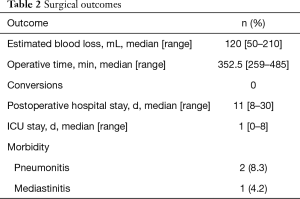
Full table
Postoperative complications occurred in 3 patients (12.5%). Complications included pneumonia in 2 patients (8.3%) and mediastinitis in 1 (4.2%). The median stay in the intensive care unit was 1 d (range, 0–8 d), whereas the median postoperative hospital stay was 11 d (range, 8–30 d). There were no in-hospital mortalities.
According to final pathology reports, all patients had complete macroscopic and microscopic (R0) resection. The pathological stages are listed in Table 3. The median number of nodes removed was 19 (range, 11–30).
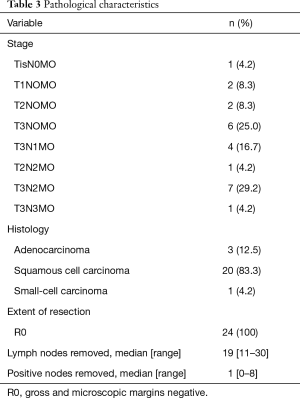
Full table
Discussion
Due to the significant reduction in cardiopulmonary complications (11,12), endoscopy-based MIE has seen widespread application in recent years. It is technically challenging to construct the tubular stomach and create an intrathoracic anastomosis under traditional endoscopy. To reduce the incidence of thoracic gastric fistula and anastomotic leaks, many surgeons choose to operate under open surgical conditions, such as with small incisions for the tubular stomach and gastroesophageal anastomosis in the neck. During recent years, the incidence of esophagogastric junction tumor has increased significantly. For these patients, cervical anastomosis may encounter many problems such as excessive anastomotic tension or insufficient gastrectomy. Therefore, it is necessary to explore more safe, maneuverable, and ergonomic techniques for minimally invasive Ivor Lewis surgery.
The intrinsic limitations, including a 2D view, reduced eye-hand coordination, and the restricted freedom of instruments, are critical difficulties for MIE in accomplishing tubular stomach production and intrathoracic anastomosis. By overcoming many of these disadvantages, the robotic surgical system can provide a viable alternative for performing this procedure.
In contrast with open surgery, conventional laparoscopy has two main difficulties in making a tubular stomach. First, the suturing is technically demanding and time-consuming, and robotic surgery can largely overcome this difficulty. The second difficulty is that trimming the stomach along the C-shaped greater curvature inside the abdomen is more difficult than achieving the equivalent operation along the stretched curvature in open procedures. We used two techniques to adjust the direction of the stapler to keep the cutting edge parallel to the greater curvature during tubularization of the stomach. One was to use polyester tape to pull the stomach without damage, and then carefully adjust the relative position of the stapler and stomach to obtain a tubular stomach with the desired width. The second was to select the assistant port under the right costal margin and robotic arm 1 port in the left abdomen as the entrance of the linear stapler to ensure that the cutting direction was the same as the direction of the greater gastric curve.
According to the literature, robotic intrathoracic anastomosis can be completed by hand-sewn, linear, and circular staplers (13-17). In the early stage of the Ivor Lewis RAMIE learning curve, we mainly used the linear stapler to perform a semi-mechanical side-to-side anastomosis. After trying 12 cases, we changed to use the circular stapler for two reasons. One was that the patients we included were diagnosed with lower esophageal cancer or esophagogastric junction tumor, which needed partial proximal gastrectomy, while the side-to-side anastomosis required a longer tubular stomach than the end-to-side anastomosis required. The second reason was that anastomotic leakage occurred in two of our 12 patients. We believe that this may have been due to the need for a longer length of the esophageal end in the side-to-side anastomosis, which may result in the poor blood supply of the anastomosis. Moreover, after end-to-side anastomosis with a circular stapler, the tissues that underwent purse-string suturing and gastrostomy were all removed, thereby reducing the risk of anastomotic complications caused by complicated operations.
The site of the small incision that introduced the stapler was also an important consideration to facilitate anastomosis construction. According to our experience, it is more convenient to adjust the angle between the stapler and the anvil when the stapler is introduced at a lower position in the chest. So, we chose the floating rib to make the small incision, which had a larger gap between the ribs to allow for smooth placement of the stapler. Also, the small incision was generally selected posterior to the scapular line. If located too far forward, it would be liable to collide with the robotic arms; if located too far backward, it would only allow for a limited range of motion due to the vertebrae.
In brief, we described our initial experience in reconstruction of the alimentary tract in the Ivor Lewis RAMIE. Our clinical data indicate that robot-assisted surgery may be a safe and feasible alternative to open and endoscopic Ivor Lewis Esophagectomy, partly owing to the accuracy in manipulation and ergonomic advantage in the robotic surgeries. Nevertheless, because of the limitations in our study, which included a small sample size and short follow-up time, it is advisable that larger comparative studies be conducted to further analyze the role of Ivor Lewis RAMIE on safety, effectiveness, and long-term survival.
Acknowledgments
Funding: This study was supported by grants from Chengdu City Science and Technology Project of China (No. 0040205301E42) and the National Key Research Project of China (No. 2017YFC0113502).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Ethics Committee of West China Hospital, Sichuan University (No. 2017-239). Written informed consents were obtained from all patients.
References
- Guo W, Ma X, Yang S, et al. Combined thoracoscopic-laparoscopic esophagectomy versus open esophagectomy: a meta-analysis of outcomes. Surg Endosc 2016;30:3873-81. [Crossref] [PubMed]
- Kauppila JH, Helminen O, Kyto V, et al. Short-Term Outcomes Following Minimally Invasive and Open Esophagectomy: A Population-Based Study from Finland and Sweden. Ann Surg Oncol 2018;25:326-32. [Crossref] [PubMed]
- Seesing MFJ, Gisbertz SS, Goense L, et al. A Propensity Score Matched Analysis of Open Versus Minimally Invasive Transthoracic Esophagectomy in the Netherlands. Ann Surg 2017;266:839-46. [Crossref] [PubMed]
- Takeuchi H, Miyata H, Ozawa S, et al. Comparison of Short-Term Outcomes Between Open and Minimally Invasive Esophagectomy for Esophageal Cancer Using a Nationwide Database in Japan. Ann Surg Oncol 2017;24:1821-7. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Bodner J, Wykypiel H, Greiner A, et al. Early experience with robot-assisted surgery for mediastinal masses. Ann Thorac Surg 2004;78:259-65; discussion 265-6. [Crossref] [PubMed]
- Ruurda JP, van Vroonhoven TJ, Broeders IA. Robot-assisted surgical systems: a new era in laparoscopic surgery. Ann R Coll Surg Engl 2002;84:223-6. [Crossref] [PubMed]
- Zhang H, Chen L, Geng Y, et al. Modified anastomotic technique for thoracolaparoscopic Ivor-Lewis esophagectomy: early outcomes and technical details. Dis Esophagus 2017;30:1-5.
- Zhang H, Chen L, Wang Z, et al. The Learning Curve for Robotic McKeown Esophagectomy in Patients With Esophageal Cancer. Ann Thorac Surg 2018;105:1024-30. [Crossref] [PubMed]
- Gao Y, Wang Y, Chen L, et al. Comparison of open three-field and minimally-invasive esophagectomy for esophageal cancer. Interact Cardiovasc Thorac Surg 2011;12:366-9. [Crossref] [PubMed]
- Ikeguchi M, Fukumoto Y. Prognostic Benefits of Thoracoscopic Esophagectomy for Thoracic Esophageal Squamous Cell Carcinomas. Chirurgia (Bucur) 2016;111:313-7. [PubMed]
- Bonavina L, Scolari F, Aiolfi A, et al. Early outcome of thoracoscopic and hybrid esophagectomy: Propensity-matched comparative analysis. Surgery 2016;159:1073-81. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Hawn MT. Technical aspects and early results of robotic esophagectomy with chest anastomosis. J Thorac Cardiovasc Surg 2013;145:90-6. [Crossref] [PubMed]
- Diez Del Val I, Loureiro Gonzalez C, Larburu Etxaniz S, et al. Contribution of robotics to minimally invasive esophagectomy. J Robot Surg 2013;7:325-32. [Crossref] [PubMed]
- Hodari A, Park KU, Lace B, et al. Robot-Assisted Minimally Invasive Ivor Lewis Esophagectomy With Real-Time Perfusion Assessment. Ann Thorac Surg 2015;100:947-52. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP. Robotic-assisted minimally invasive esophagectomy: the Ivor Lewis approach. Thorac Surg Clin 2014;24:211-22. vii. [Crossref] [PubMed]
- Trugeda S, Fernandez-Diaz MJ, Rodriguez-Sanjuan JC, et al. Initial results of robot-assisted Ivor-Lewis oesophagectomy with intrathoracic hand-sewn anastomosis in the prone position. Int J Med Robot 2014;10:397-403. [Crossref] [PubMed]