
Correlation between thyroid transcription factor-1 expression, immune-related thyroid dysfunction, and efficacy of anti-programmed cell death protein-1 treatment in non-small cell lung cancer
Introduction
Anti-programmed cell death protein-1 (anti-PD-1) antibody, nivolumab or pembrolizumab, prolongs overall survival (OS) in patients with advanced or recurrent non-small cell lung cancer (NSCLC) (1-4).
The interruption of PD-1 signaling by anti-PD-1 antibody reactivates T-cell–mediated antitumor immunity, but alteration of the immune signaling pathway results in various immune-related adverse events (irAEs). In melanoma patients who received immunotherapy including anti-PD-1 treatment, skin irAEs, especially vitiligo, have correlated with clinical response or survival (5-9). This association is thought to be caused by shared antigens between melanoma cells and normal melanocytes (7,8).
Recent studies have suggested a correlation between irAEs, including immune-related thyroid dysfunction (irTD), and the superior efficacy of anti-PD-1 treatment in NSCLC (10-14). Previously, certain cancer-testis antigens that are expressed in NSCLC were reported to have amino acid sequence homology with thyroid autoantigens, and thyroid dysfunction occurred by cross-reaction (15-17). These findings suggest that irTD is caused by shared antigen between NSCLC and the thyroid, and may indicate anti-NSCLC immunity in anti-PD-1 treatment.
The lung and thyroid differentiate from the endoderm and embryologically they are similar in origin. Thyroid transcription factor-1 (TTF-1), which is known to be a member of the homeodomain-containing transcription factor family and controls embryonic development and morphogenesis of the thyroid and lung, expresses in both organs, including NSCLC (18-22). In this context, we hypothesized that TTF-1 expression in NSCLC, which could be a shared antigen with the thyroid, might correlate with irTD incidence and anti-PD-1 treatment efficacy. To explore this hypothesis, we conducted a retrospective analysis.
Methods
Patient selection
Patients with advanced or recurrent NSCLC who received anti-PD-1 antibody, nivolumab or pembrolizumab, at the Cancer Institute Hospital of Japanese Foundation for Cancer Research between December 2015 and June 2017 were identified, and those evaluated for TTF-1 expression were analyzed.
Data collection and assessment
We reviewed subjects’ medical records. Clinical information, including patient characteristics; laboratory, radiologic, and pathologic findings; anticancer therapy history; and response to treatment, were collected retrospectively. TTF-1 in biopsy specimens was immunostained using a mouse monoclonal antibody (clone 8G7G3/1, 1:100; Dako, Santa Clara, CA, USA), as described previously (23). TTF-1 immunostaining results were based on nuclear staining of neoplastic cells. Tumors were considered negative expression if staining was found in <5% of neoplastic cells and positive expression if in ≥5% (24). In this analysis, TTF-1 expression of ≥5% was classified as positive uniformly, regardless of the intensity of staining. Thyroid function tests, including thyroid-stimulating hormone (TSH), free thyroxine (fT4), and free triiodothyronine (fT3), were measured before treatment and monthly after the first administration of anti-PD-1 antibody. We defined ≥2 successive abnormal TSH levels during anti-PD-1 treatment as indicating irTD. All subjects underwent computed tomography every 2–3 months with a few exceptions. Tumors were assessed according to the Response Evaluation Criteria in Solid Tumors version 1.1 (25). Median follow-up was defined as the median length of time between the first administration of anti-PD-1 antibody and death or last survival. We defined progression-free survival (PFS) as the length of time between the first administration of anti-PD-1 antibody and death or disease progression, and OS as the length of time between the first administration of anti-PD-1 antibody and death. Patients were censored when information on time to event was not available due to loss to follow-up or non-occurrence of outcome event before the end of follow-up. PFS, OS, objective response rate (ORR), and disease control rate (DCR) to anti-PD-1 treatment were determined using treating investigator tumor assessments. We assessed retrospectively the irTD incidence and anti-PD-1 treatment efficacy in patients positive or negative for TTF-1. Follow-up ended on August 31, 2017.
Statistical analysis
We compared baseline differences in patient characteristics between subgroups using Fisher’s exact test or Student’s t-test. ORR, DCR, and irTD incidence were compared with Fisher’s exact test. Among baseline patient characteristics, the risk factors for irTD were explored using univariate and subsequent multivariate logistic regression analysis. Factors with P<0.20 on univariate analyses were included for multivariate logistic regression analysis. PFS and OS curves were estimated using the Kaplan-Meier method and the differences between subgroups were compared by the log-rank test. All statistical tests were two-sided and P<0.05 was considered statistically significant. Statistical analyzes were performed using EZR on R commander version 1.36 (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (26).
Results
Patient characteristics
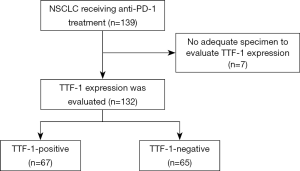
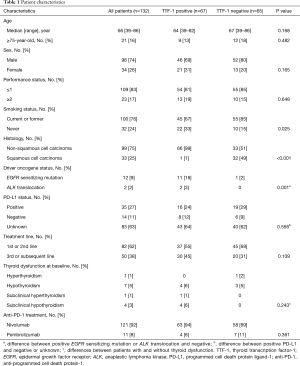
A total of 139 patients with advanced or recurrent NSCLC received anti-PD-1 treatment. Of 132 patients evaluated for TTF-1 expression, 67 (51%) and 65 (49%) were positive and negative for TTF-1, respectively (Figure S1). Patient characteristics are shown in Table 1. The median patient age was 66 years. Most patients were male, had a performance status (PS) score of ≤1, were current or former smokers, and had undergone ≤1 regimen of previous systemic therapy. A total of 14 (11%) patients had the EGFR sensitizing mutation or ALK translocation. Nivolumab and pembrolizumab were given in 121 (92%) and 11 (8%) patients, respectively. Never smoker, non-squamous (NSQ) cell carcinomas, and driver oncogene-positive patients were more frequent in the TTF-1-positive compared to the TTF-1-negative NSCLC groups. At baseline, thyroid dysfunction was observed in 13 patients.
Full table
Association between TTF-1 expression and IrTD incidence
Among 132 patients, irTD was observed in 19 (14%); 6 and 13 were TTF-1-positive and TTF-1-negative, respectively. Of the 19 cases with irTD, there were 10 grade 1 and 9 grade 2. The irTD incidence in TTF-1-positive and TTF-1-negative NSCLCs was 9% and 20%, respectively (P=0.086). Particularly, the irTD incidence was significantly higher in the NSQ cell carcinomas patients negative for TTF-1 (30%) than those positive for TTF-1 (9%; P=0.010) (Table 2). The incidence of irTD, regardless of grade, tended to be higher in TTF-1-positive than in TTF-1-negative patients (Tables S1,S2). On the other hand, the incidence of irAE other than irTD was not different between the TTF-1-positive and TTF-1-negative NSCLC groups (Table S3).

Full table

Full table

Full table

Full table
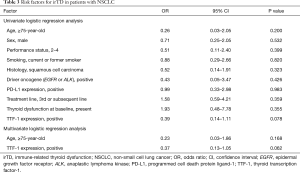
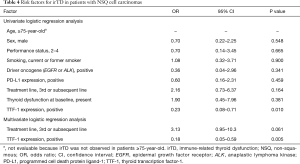
We evaluated the risk factors for irTD. In patients with NSCLC, although TTF-1 expression and age were candidate risk factors on univariate logistic regression analysis, these factors did not show statistically significant difference on multivariate analysis (Table 3). In patients with NSQ cell carcinomas, however, TTF-1 expression and treatment line were candidate risk factors on univariate logistic regression analysis, and TTF-1 expression was identified as a significant factor on multivariate analysis [OR, 0.18; 95% confidence interval (CI), 0.05–0.59; P=0.005] (Table 4).
Full table
Full table
Correlation between TTF-1 expression, IrTD, and anti-PD-1 treatment efficacy
One hundred five patients (80%) had progression events and 54 (41%) had died at the time of analysis (median follow-up, 7.6 months; range, 0.2–19.8 months).
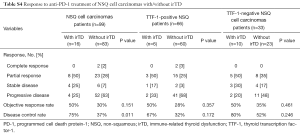
Correlation between TTF-1 expression and irTD and tumor response is shown in Table 5. In NSCLC, the ORR of patients with/without irTD was 47% and 29% respectively (P=0.181). The DCR was significantly higher in patients with irTD (79%) than in those without irTD (39%; P=0.002). In the TTF-1-positive NSCLC group, no significant differences in ORR and DCR were observed between patients with and without irTD. However, in the TTF-1-negative NSCLC group, DCR was significantly higher in patients with irTD (85%) than in those without irTD (48%; P=0.027). Furthermore, also in the NSQ cell carcinomas group, the development of irTD was associated with a good response (Table S4).

Full table
Full table
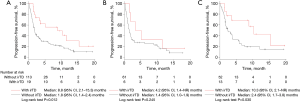
Kaplan-Meier analysis of PFS is shown in Figure 1. Median PFS was significantly longer in NSCLC patients with irTD than in those without irTD (8.8 months; 95% CI, 2.1–15.3 months vs. 1.8 months; 95% CI, 1.4–2.4 months; P=0.012). In the TTF-1-positive NSCLC group, no significant differences in PFS were observed between patients with and without irTD. In the TTF-1-negative NSCLC group, however, there was a significant difference in median PFS between these two groups [10.3 months; 95% CI, 2.1–not reached (NR) months vs. 2.4 months; 95% CI, 1.7–3.8 months; P=0.030].
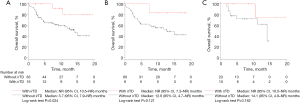
Kaplan-Meier analysis of OS is shown in Figure 2. Median OS was significantly longer in NSCLC patients with irTD than in those without irTD (NR; 95% CI, 10.5–NR months vs. 14.1 months; 95% CI, 9.4–NR months; P=0.011). In the TTF-1-positive and TTF-1-negative NSCLC groups, median OS tended to be longer in patients with irTD than in those without irTD. This tendency was remarkable in the TTF-1-negative NSCLC group (NR; 95% CI, 10.5–NR months vs. 14.1 months; 95% CI, 9.4–NR months; P=0.062).

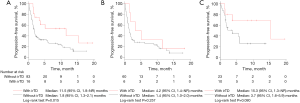
We also investigated the relationship between irTD and survival in patients with NSQ cell carcinomas (Figures S2,S3). PFS and OS were significantly longer in NSQ cell carcinomas patients with irTD than in those without irTD. As in the population of NSCLC, PFS and OS tended to be long particularly in TTF-1-negative NSQ cell carcinomas patients with irTD.
Discussion
To our knowledge, this is the first report to refer to the association between TTF-1 expression of NSCLC, irTD, and anti-PD-1 treatment efficacy. Although the shared antigen is important for the immune reaction, there are few reports on the antigen related to irAE and anti-PD-1 treatment efficacy. Our analysis suggested that the incidence of irTD might be high in patients with TTF-1-negative NSCLC. Furthermore, in such patients, the development of irTD was correlated strongly with superior response or survival with anti-PD-1 treatment.
As a mechanism of irAEs, increasing T-cell activity against shared antigens that are present in tumors and healthy tissue is proposed (27,28). Although TTF-1 is known as a protein commonly expressed in the lung and thyroid, it is unknown whether TTF-1 expression of NSCLC correlates with irTD development in anti-PD-1 treatment. We assumed that irTD might develop frequently in patients with TTF-1-positive NSCLC, but surprisingly, the result was opposite to our hypothesis. As a reason for the opposite correlation between TTF-1 expression and irTD development, sustained exposure of shared antigens may be considered. During chronic infection or cancer, T-cell exhaustion, which is a state of T-cell dysfunction defined by poor effector function and sustained expression of various inhibitory receptors, occurs (29). The duration and magnitude of antigenic stimulation is known as a factor determining the severity of T-cell exhaustion (30). TTF-1 is found also in normal alveolar type II cells and thyroid tissue. In TTF-1-positive NSCLC, the recognition of TTF-1 as a cancer antigen may increase the magnitude of antigenic stimulation. We speculate that an immune reaction induced by anti-PD-1 treatment may be unlikely to occur when the expression and exposure of shared antigen are high because T-cell exhaustion occurs strongly. Recently, the frequency of irTD has been suggested to be higher in squamous cell carcinoma (SQ) than NSQ cell carcinomas according to KEYNOTE-407 (31) and KEYNOTE-189 (32) (phase 3 trials of combination of pembrolizumab and chemotherapy for SQ-NSCLC and NSQ-NSCLC, respectively). Hypothyroidism was observed in 7.9%, hyperthyroidism in 7.2%, and thyroiditis in 1.1% in KEYNOTE-407, compared with 6.7%, 4.0%, and 0.2% in the KEYNOTE-189, respectively. The frequency of irTD tended to be high in the KEYNOTE-407. These reports are consistent with our findings.
Increasing levels of preexisting autoantibodies were reported as another mechanism of irAEs (33). Recently, the association between irTD and anti-thyroid antibodies, such as anti-thyroglobulin antibodies or anti-thyroid peroxidase antibody detected before anti-PD-1 treatment, was reported (10,34). However, these reports do not explain the full mechanism of irTD because there are irTDs without anti-thyroid antibodies. We have not examined for anti-thyroid antibodies, but the frequency of thyroid dysfunction at baseline was not different between TTF-1-positive and TTF-1-negative patients. Particularly in patients with NSQ cell carcinomas, however, TTF-1 expression of tumor was identified as a significant risk factor for irTD. This suggested that the characteristics of the tumor itself may affect the irTD incidence. Recently, a tumor-specific irAE pattern has been reported (35), which supports our suggestion.
Although TTF-1 has been expressed in various types of lung cancer, it is reported frequently in NSQ-NSCLC (36). In this analysis, histology and TTF-1 expression were evaluated by biopsy specimens in most subjects. When the tumor has a heterogeneity, evaluation of biopsy specimens may be insufficient to assess whole tumor characteristics. In addition, the histological subgroup was defined by the pathologist at the initial diagnosis in this analysis. In some cases, TTF-1 was not stained at diagnosis and TTF-1 immunostaining was added later. Therefore, we analyzed the population of NSCLCs and NSQ cell carcinomas. Both analyses suggested similar results that TTF-1 expression might be correlated with irTD incidence, and PFS or OS tended to be long in TTF-1-negative patients with irTD.
There are some limitations in this study. First, this is a retrospective analysis of a small sample size from a single institution and the background of subjects is not unified. Because programmed cell death protein ligand-1 (PD-L1) expression was evaluated in <40% of our patients, obtaining the correlation between PD-L1 status and efficacy was difficult. Our study included patients with EGFR sensitizing mutation or ALK translocation and those with PS score of ≥2 for whom immunotherapy is controversial. The number of previous chemotherapies of the participant varies, and there are some differences in the timing of treatment efficacy evaluation. These can make the data on efficacy less robust. Second, we did not consider the timing of irTD onset. Although irTD is an adverse event that occurs early in treatment, there may be lead-time bias. Therefore, we investigated the relationship between TTF-1 expression and irTD in population with treatment duration within 3 months and of 3 months or more. In either population, the frequency of irTD tended to be lower in TTF-1-positive patients. These findings suggest that TTF-1 expression is related to the occurrence of irTD regardless of the treatment period (Tables S5,S6). Third, we assumed that the correlation between TTF-1 expression and irTD incidence was caused by shared antigens, but we did not confirm the actual cross-reaction. However, we demonstrated that TTF-1 is a representative shared antigen and a promising candidate for understanding irTD and the effect of anti-PD-1 treatment. We speculate that an immune reaction induced by anti-PD-1 treatment to TTF-1 positive NSCLC is suppressed to some degree because of T-cell exhaustion as a result of immune tolerance to normal organs. Future investigation of shared antigens including TTF-1 might contribute to development in anti-PD-1 treatment, leading to better patient selection.

Full table

Full table
In conclusion, our study suggested that TTF-1 expression in NSCLC might correlate with irTD and anti-PD-1 treatment efficacy. Further studies are needed to validate our findings.
Acknowledgments
The authors thank all the study participants who provided clinical data for the analysis.
Footnote
Conflict of Interest: M Nishio received research funding from Novartis, ONO Pharmaceutical, Chugai Pharmaceutical, Bristol-Myers Squibb, TAIHO Pharmaceutical, Eli Lilly, Pfizer, Astellas Pharma and AstraZeneca, and honoraria from Pfizer, Bristol-Myers Squibb, ONO Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, TAIHO Pharmaceutical and AstraZeneca. A Horiike received research funding from Chugai Pharmaceutical, Quintiles, MSD oncology, and Abbvie, and honoraria from Chugai Pharmaceutical, Eli Lilly, AstraZeneca, Pfizer, Boehringer Ingelheim, ONO Pharmaceutical. Y Ishikawa received research funding from Daiichi Sankyo, and honoraria from Bristol-Myers Squibb, MSD Oncology, ONO Pharmaceutical, Novartis. N Yanagitani received honoraria from MSD Oncology, Bristol-Myers Squibb, ONO Pharmaceutical. The other authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the institutional review board of The Cancer Institute Hospital of Japanese Foundation for Cancer Research (No. 2018-1125).
References
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540-50. [Crossref] [PubMed]
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 2016;375:1823-33. [Crossref] [PubMed]
- Teulings HE, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33:773-81. [Crossref] [PubMed]
- Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in Resected and Unresectable Metastatic Melanoma: Characteristics of Immune-Related Adverse Events and Association with Outcomes. Clin Cancer Res 2016;22:886-94. [Crossref] [PubMed]
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab Cutaneous Adverse Events and Their Association With Disease Progression. JAMA Dermatol 2015;151:1206-12. [Crossref] [PubMed]
- Hua C, Boussemart L, Mateus C, et al. Association of Vitiligo With Tumor Response in Patients With Metastatic Melanoma Treated With Pembrolizumab. JAMA Dermatol 2016;152:45-51. [Crossref] [PubMed]
- Nakamura Y, Tanaka R, Asami Y, et al. Correlation between vitiligo occurrence and clinical benefit in advanced melanoma patients treated with nivolumab: A multi-institutional retrospective study. J Dermatol 2017;44:117-22. [Crossref] [PubMed]
- Osorio JC, Ni A, Chaft JE, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockage in patients with non-small-cell lung cancer. Ann Oncol 2017;28:583-9. [PubMed]
- Hasan Ali O, Diem S, Markert E, et al. Characterization of nivolumab-associated skin reactions in patient with metastatic non-small cell lung cancer. Oncoimmunology 2016;5:e1231292. [Crossref] [PubMed]
- Haratani K, Hayashi H, Chiba Y, et al. Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol 2018;4:374-8. [Crossref] [PubMed]
- Teraoka S, Fujimoto D, Morimoto T, et al. Early Immune-Related Adverse Events and Association with Outcome in Advanced Non-Small Cell Lung Cancer Patients Treated with Nivolumab: A Prospective Cohort Study. J Thorac Oncol 2017;12:1798-805. [Crossref] [PubMed]
- Sato K, Akamatsu H, Murakami E, et al. Correlation between immune-related adverse events and efficacy in non-small cell lung cancer treated with nivolumab. Lung Cancer 2018;115:71-4. [Crossref] [PubMed]
- Gure AO, Chua R, Williamson B, et al. Cancer-testis genes are coordinately expressed and are markers of poor outcome in non-small cell lung cancer. Clin Cancer Res 2005;11:8055-62. [Crossref] [PubMed]
- Grunwald C, Koslowski M, Arsiray T, et al. Expression of multiple epigenetically regulated cancer/germline genes in nonsmall cell lung cancer. Int J Cancer 2006;118:2522-8. [Crossref] [PubMed]
- Vita R, Guarneri F, Agah R, et al. Autoimmune thyroid disease elicited by NY-ESO-1 vaccination. Thyroid 2014;24:390-4. [Crossref] [PubMed]
- Lazzaro D, Price M, de Felice M, et al. The transcription factor TTF-1 is expressed at the onset of thyroid and lung morphogenesis and in restricted regions of the foetal brain. Development 1991;113:1093-104. [PubMed]
- Ikeda K, Clark JC, Shaw-White JR, et al. Gene structure and expression of human thyroid transcription factor-1 in respiratory epithelial cells. J Biol Chem 1995;270:8108-14. [Crossref] [PubMed]
- Minoo P, Hamdan H, Bu D, et al. TTF-1 regulates lung epithelial morphogenesis. Dev Biol 1995;172:694-8. [Crossref] [PubMed]
- Acebrón A, Aza-Blanc P, Rossi DL, et al. Congenital human thyroglobulin defect due to low expression of the thyroid-specific transcription factor TTF-1. J Clin Invest 1995;96:781-5. [Crossref] [PubMed]
- Stenhouse G, Fyfe N, King G, et al. Thyroid transcription factor 1 in pulmonary adenocarcinoma. J Clin Pathol 2004;57:383-7. [Crossref] [PubMed]
- Inamura K, Satoh Y, Okumura S, et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol 2005;29:660-5. [Crossref] [PubMed]
- Inamura K, Takeuchi K, Togashi Y, et al. EML4-ALK lung cancers are characterized by rare other mutations, a TTF-1 cell lineage, an acinar histology, and young onset. Mod Pathol 2009;22:508-15. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant 2013;48:452-8. [Crossref] [PubMed]
- Johnson DB, Balko JM, Compton ML, et al. Fulminant Myocarditis with Combination Immune Checkpoint Blockade. N Engl J Med 2016;375:1749-55. [Crossref] [PubMed]
- Byrne EH, Fisher DE. Immune and molecular correlates in melanoma treated with immune checkpoint blockade. Cancer 2017;123:2143-53. [Crossref] [PubMed]
- Wherry EJ. T cell exhaustion. Nat Immunol 2011;12:492-9. [Crossref] [PubMed]
- Yi JS, Cox MA, Zajac AJ. T-cell exhaustion: characteristics, causes and conversion. Immunology 2010;129:474-81. [Crossref] [PubMed]
- Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus Chemotherapy for Squamous Non-Small-Cell Lung Cancer. N Engl J Med 2018;379:2040-51. [Crossref] [PubMed]
- Gandhi L, Rodrigues-Abreu D, Gadgeel S, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med 2018;378:2078-92. [Crossref] [PubMed]
- Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378:158-68. [Crossref] [PubMed]
- Kobayashi T, Iwama S, Yasuda Y, et al. Patients With Antithyroid Antibodies Are Prone To Develop Destructive Thyroiditis by Nivolumab: A Prospective Study. J Endocr Soc 2018;2:241-51. [Crossref] [PubMed]
- Khoja L, Day D, Wei-Wu Chen T, et al. Tumor- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: a systematic review. Ann Oncol 2017;28:2377-85. [Crossref] [PubMed]
- Tan D, Li Q, Deeb G, et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol 2003;34:597-604. [Crossref] [PubMed]


