
Fluid administration during lung resection: what is the optimum?
It is well known that liberal fluid administration during and following lung resection is associated with postoperative pulmonary complications (1,2). Our publication in 2015 reported a frightening pulmonary complication rate of 56% in a cohort of 139 patients undergoing a variety of lung resections from lobectomy to pneumonectomy and a threshold of 6 mL/kg/h for total fluid administration in order to decrease/avoid pulmonary complications.
Wu et al., published their detailed analysis of 446 patients who underwent minimally invasive lobectomy between May 2016 and April 2017 titled “Effects of Intraoperative Fluid Management on Postoperative Outcomes following Lobectomy” (3). The cohort is a highly selected subgroup of patients with strict exclusion criteria. All patients had a minimally invasive lobectomy, forced expiratory volume in 1 second (FEV1) was 90% on average and patients younger than 18, older than 70, those with a body mass index (kg/m2) less than 18.5 and more than 28 were excluded. Current series reflects a contemporary minimally invasive thoracic surgical practice focused only on lobectomy in patients with perfect preoperative clinical condition coupled with a short anesthesia time (around 160 min) and limited intraoperative bleeding (<100 mL). There were also no patients with neoadjuvant therapy or with extended resections. As a result of good candidates and perfect surgical technique, there was no perioperative mortality. However, in this highly selected subgroup of patients, postoperative pulmonary complication rate was a striking 38.6% (201 pulmonary complications in 172 patients)! This shows the trauma caused by removal of a pulmonary lobe from the body, regardless of the patient condition, number of incisions or perfection of the technique of lung resection.
The authors concluded that restrictive (≤9.4 mL/kg/h), moderately liberal (11.8–14.2 mL/kg/h) and liberal (14.2 mL/kg/h) total fluid administration was associated with higher pulmonary complication rate. Pulmonary complications were higher if colloids were not infused or at a restricted rate (0–3.8 mL/kg/h) rate. Moderate (>9.4–11.8 mL/kg/h) rate of total fluid and colloid (>3.8 mL/kg/h) administration were associated with less pulmonary complications.
The authors should be commended for their detailed analysis and search for a window of safe fluid administration rate. How should we translate this data in the current body of evidence to our practice?
The data shows that safe total fluid administration rate of 658–826 mL/h is the optimum for a 70 kg patient during a lobectomy. Addition of a minimum of 266 mL/h colloid infusion in the same patient is associated with a lower pulmonary complication rate as well. This is practically 1 L/h fluid administration.
The difference between previous studies and current study is obvious (Table 1) (1-4). Two of the large series in 2006 (n=1,222) and 2009 (n=558) included patients who had thoracotomy (1,5). In the prior series, the total amount of fluids infused was 1,533 mL, and pulmonary complication rate was 18.3% (1). In the latter series, infusion rate was 5.8±2.9 mL/kg/h (5).
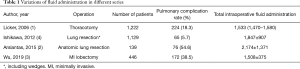
Full table
In the current study, bleeding is less than 100 cc and insensible loss through a small incision is almost none. However, despite a liberal amount of fluid infusion in all groups, 8 patients out of 446 (1.8%) had acute kidney injury (3). In our series where the average amount of fluid infused was almost half the amount in the series by Wu et al., only 2 out of 139 (1.4%) patients experienced renal failure needing temporary dialysis with no long-term kidney injury (2). It has been shown that the notion of targeting normovolemia (1.5 mL/kg/h plus intraoperative losses) was adequate in terms of maintaining normal creatinine levels (6).
It has been shown that albumin levels are higher in VATS patients in the postoperative period (7). As a contrary outcome to the Wu et al.’s study, Ishikawa and colleagues showed that colloids increased postoperative acute kidney injury, resulting in a higher rate of pulmonary complications and prolonged hospitalization (4). Thus, the need for colloids intra- and postoperatively for patients undergoing VATS anatomic lung resections is questionable.
We know that inflammatory markers are much less with minimally invasive surgery. Interleukin (IL)-6, IL-8 and IL-10 were all found to be lower after VATS compared with thoracotomy and IL-6 is well known to be associated with pulmonary edema (7,8). Thus, study by Wu et al. has a cohort of patients with much less inflammation and tissue trauma which shows that much more liberal fluid management is associated with a better outcome.
It was shown that being underweight (BMI <18.5) or severely overweight (BMI ≥40) was associated with increased risk of pulmonary complications and any postoperative complications respectively (9). Wu et al.’s study also excluded underweight and obese patients.
Current study shows that:
- MI VATS lobectomies can be safely performed without any mortality;
- The cohort is very different than a western population and include younger patients (average age 58) and more females (273 out of 446) with a lower smoking rate (25–30%) and an average BMI of 23;
- Liberal use of fluids (almost 1 L/h) is safe in this cohort, however pulmonary complication rate of 38.6% in this cohort is relatively higher than previous studies in the literature (Table 1) with patients who underwent thoracotomy.
We very well know from other specialties that even mild fluid overload has adverse physiologic effects on lung function and leads to worsened pulmonary and non-pulmonary outcomes after surgery and during the resolution phase of acute respiratory distress syndrome (10-12).
In conclusion, current study is valuable in terms of identifying a safe margin for fluid infusion in MI lobectomy patients, however the fluid strategy can’t be translated to every lung resection patient. It is important to protect the lungs from fluid overload and adjust our strategy based on patient factors.
Every patient has his/her own optimum fluid balance!
Acknowledgments
None.
Footnote
Conflicts of Interest: HF Batirel: Johnson & Johnson—Honoraria, Consultancy.
References
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Arslantas MK, Kara HV, Tuncer BB, et al. Effect of the amount of intraoperative fluid administration on postoperative pulmonary complications following anatomic lung resections. J Thorac Cardiovasc Surg 2015;149:314-20, 321.e1.
- Wu Y, Yang R, Xu J, et al. Effects of Intraoperative Fluid Management on Postoperative Outcomes following Lobectomy. Ann Thorac Surg 2019;107:1663-9. [Crossref]
- Ishikawa S, Griesdale DE, Lohser J. Acute kidney injury after lung resection surgery: incidence and perioperative risk factors. Anesth Analg 2012;114:1256-62. [Crossref] [PubMed]
- Licker M, Fauconnet P, Villiger Y, et al. Acute lung injury and outcomes after thoracic surgery. Curr Opin Anaesthesiol 2009;22:61-7. [Crossref] [PubMed]
- Assaad S, Popescu W, Perrino A. Fluid management in thoracic surgery. Curr Opin Anaesthesiol 2013;26:31-9. [Crossref] [PubMed]
- Jones RO, Anderson NH, Murchison JT, et al. Innate immune responses after resection for lung cancer via video-assisted thoracoscopic surgery and thoracotomy. Innovations (Phila) 2014;9:93-103; discussion 103. [PubMed]
- Rassler B. Role of α- and β-adrenergic mechanisms in the pathogenesis of pulmonary injuries characterized by edema, inflammation and fibrosis. Cardiovasc Hematol Disord Drug Targets 2013;13:197-207. [Crossref] [PubMed]
- Williams T, Gulack BC, Kim S, et al. Operative Risk for Major Lung Resection Increases at Extremes of Body Mass Index. Ann Thorac Surg 2017;103:296-302. [Crossref] [PubMed]
- Licker M, de Perrot M, Spiliopoulos A, et al. Risk factors for acute lung injury after thoracic surgery for lung cancer. Anesth Analg 2003;97:1558-65. [Crossref] [PubMed]
- Yao S, Mao T, Fang W, et al. Incidence and risk factors for acute lung injury after open thoracotomy for thoracic diseases. J Thorac Dis 2013;5:455-60. [PubMed]
- Seeley EJ. A dry lung is a happy lung: more supporting evidence. J Thorac Cardiovasc Surg 2015;149:321-2. [Crossref] [PubMed]


