
A novel air leak test using surfactant: a step forward or a bubble that will burst?
An intraoperative alveolar air leak is one of the most common complications during lung resections. Many air leaks will resolve spontaneously within 48 hours however a significant proportion of air leaks can be prolonged. A prolonged air leak (PAL) is defined in general as air leakage lasting more than 5 days. The incidence of a PAL is reported to be 8–10% of patients undergoing lung resections (1-4). Patients with PAL have a higher incidence of postoperative complications, a prolonged length of stay and increased medical costs (2-4). Several patient characteristics have been identified as independently associated with an increased risk for a prolonged leak following lung resections: male sex, smoking history, body mass index (BMI) ≤25 kg/m2, Medical Research Council dyspnea score greater than 1, chronic obstructive pulmonary disease (COPD), lower forced expiratory volume in 1 second (FEV1) and diffusing capacity of the lungs for carbon monoxide (DLCO) <80% are associated with an increased risk of PAL after lung resection (1-4). In addition, operative factors, such as upper lobe resections and the presence of pleural adhesions are associated with PALs (2,3).
A common theme among these risk factors, especially a low FEV1 and low DLCO, is that these are associated with the emphysema-phenotype of COPD. In an important study utilizing computed tomography (CT) quantification of emphysema, the higher the percentage of emphysema was the higher the risk of PAL following lung resection (5). CT quantification of emphysema was the strongest predictor of PAL (5). Furthermore, the highest risk for PALs occurs in patients undergoing lung volume reduction surgery for emphysema with a reported incidence of 24–46%.
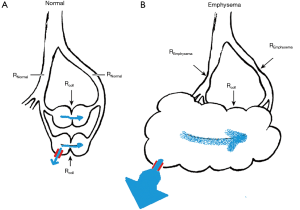
The physiologic principle linking emphysema to an increased risk for PAL is likely related to collateral ventilation (6,7). Collateral ventilation is defined as “the ventilation of alveolar structures through passages or channels that bypass the normal airways” (7). In structurally normal lungs collateral airflow resistance is greater than airway resistance (Figure 1A) (7). However, in emphysema, airway resistance can by far exceed collateral airflow resistance (Figure 1B) (7). In emphysema the lower resistance pathways of collateral ventilation can cause air to flow preferentially through collateral pathways and when incomplete fissures are present collateral ventilation can connect airspaces of an entire lung (Figure 1B) (7-11). In the setting of pleuro-parenchymal tears/defects (as can occur during lung resections) of an emphysematous lung, transpleural airflow out through the tear can be a pathway of least resistance (Figure 1B) (8-11). When a pleuro-parenchymal tear/defect occurs in a lung with significant emphysema there can be complete transpleural exhalation via the air leak (8-11).
In light of the significant morbidity and cost associated with PALs the best strategy would be prevention. Key elements for the prevention of PALs is identifying at risk patients and identifying and quantifying intraoperative alveolar air leaks and to address those that are significant (and associated with PAL) during the index surgery.
In general, the intraoperative assessment for an air leak is performed by a water submersion test (WST). The WST is the traditional method of evaluating air leakage during surgery and is performed by filling the thoracic cavity with saline and observe the degree of air bubbles generated during manual positive pressure ventilation.
This WST is used to detect the presence and the degree of the air leak. During this test each surgical site is graded with the lung submersed in saline solution and inflated during positive pressure ventilation; the presence or absence of air leaks is often scored as grade 0 (no leak), 1 (countable bubbles), 2 (stream of bubbles), and 3 (coalesced bubbles) (12). However, the WST is a subjective assessment and depends on the individual surgeon’s experience. Furthermore, the WST is often challenging during video-assisted thoracoscopic surgery (VATS) procedures, as the vision can be more challenging via the thoracoscope. Furthermore, with a submersion method it is often required for the lung parenchyma to be held and manipulated to identify the leak point and subsequently the lung might not be in its physiological state and it might be more challenging to identify air leaks under those circumstances. Of note, most air leaks are not at the suture lines but in the area of the fissure. In this area, the possibilities to “seal” the leakage are very limited due to the interlobar branches of the pulmonary artery. Thus, often detected air leakage during surgery has no real consequence.
Yang and Chang report on a novel air leak test using surfactant (13). The objectives of this study were to provide optimal visualization under the conditions of VATS that allows to clearly detect the air leak point. Furthermore, the substance used for the air leak detection needed to be harmless for humans and economical.
Yang and Chang utilized an ex vivo model of a normal porcine lung and generated a defined air leak by puncturing the lung with an 18-gauge needle. Under those experimental conditions the authors found that using a green colored Pluronic® F-127 solution (Called by the authors Yang’s bubble solution) was best suited for detecting the air leak. Each lobe was examined separately by instilling Yang’s bubble solution over the lung surface (instead of submersion of the lung in water, as with the WST). To examine the entire lung approximately 60 mL of Yang’s bubble solution was needed.
There are several notable limitations and open questions regarding Yang’s bubble solution that would require further careful characterization (14). In the experimental set up used a normal porcine lung was injured with an 18-gauge needle to introduce a defined air leak. This is quite different compared to the clinical situation were often patients with significant underlying structural lung disease such as COPD and emphysema undergo lung resections. In clinical practice air leaks occur in the setting of a lung resection of an often structurally abnormal lung (which is quite different from a defined puncture with an 18-gauge needle of a structurally normal lung). It is unclear how Yang’s bubble solution would perform under the conditions of a higher flow air leak then a small 18-gauge needle injury is causing in a structurally normal lung and how that might affect the ability to clearly visualize the location of the air leak.
The severity of the air leak has also important clinical implications. An air leak from a small defect such as an injury from an 18-gauge needle will likely heal by itself without needing further intervention such as oversewing or application of a sealant.
This highlights that a quantitative, objective assessment of the intraoperative air leak has importance. The air leak can be quantified by measuring the intraoperative leak during mechanical ventilation. This leak can be calculated by measuring inspiratory and expiratory tidal volumes during mechanical ventilation at the end of the procedure (15,16). Several studies showed an association between the severity of the intraoperative air leak, as assessed by the comparison of Inspiratory and expiratory tidal volumes during a structured mechanical ventilation test, and the risk of PAL (15,16). Zaraca et al. reported on a randomized controlled trial focusing on selecting patients for intraoperative sealant treatment to prevent PAL based on a quantitative intraoperative air leak assessment utilizing a standardized mechanical ventilation test (17). Mild intraoperative air leaks (<100 mL/min) were considered self-limiting and not treated. Severe intraoperative air leaks (>400 mL/min) were all treated. An intraoperative air leak between 100 and 400 mL/min was defined as moderate and constituted the entry criteria to select the study population for a prospective multicenter randomized trial on the use of a sealant. Patients with moderate intraoperative air leaks were randomized to sealant or “no treatment” control group. The mean air leakage duration was 1.60 days in the sealant group, which was significantly shorter compared to 5.04 days in the control groups (P<0.001) (17).
For the future, we need risk assessment tools for PAL derived from:
- Preoperatively available parameters based on patients’ characteristics, such as demographics (age, gender, smoking and COPD history), pulmonary function studies (FEV1 and DLCO) and quantitative chest CT analysis, to assess and quantify the degree of structurally abnormal lung and emphysema;
- From surgical characteristics such as the type of surgery and from intraoperative objective and quantitative air leak assessments.
These parameters can guide patient selection for intraoperative sealant and surgical strategies to prevent PAL.
It remains to be seen, if Yang’s bubble solution can improve the intraoperative objective air leak assessment as part of a multifaceted approach to prevent PAL in at risk patients.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Gilbert S, Maghera S, Seely AJ, et al. Identifying Patients at Higher Risk of Prolonged Air Leak After Lung Resection. Ann Thorac Surg 2016;102:1674-9. [Crossref] [PubMed]
- Brunelli A, Cassivi SD, Halgren L. Risk factors for prolonged air leak after pulmonary resection. Thorac Surg Clin 2010;20:359-64. [Crossref] [PubMed]
- Pompili C, Falcoz PE, Salati M, et al. A risk score to predict the incidence of prolonged air leak after video-assisted thoracoscopic lobectomy: An analysis from the European Society of Thoracic Surgeons database. J Thorac Cardiovasc Surg 2017;153:957-65. [Crossref] [PubMed]
- Zhao K, Mei J, Xia C, et al. Prolonged air leak after video-assisted thoracic surgery lung cancer resection: risk factors and its effect on postoperative clinical recovery. J Thorac Dis 2017;9:1219-25. [Crossref] [PubMed]
- Petrella F, Rizzo S, Radice D, et al. Predicting prolonged air leak after standard pulmonary lobectomy: computed tomography assessment and risk factors stratification. Surgeon 2011;9:72-7. [Crossref] [PubMed]
- Eberlein M, Parekh KR, Keech J, et al. Prolonged Air Leak After Lung Resection and Emphysema. Ann Thorac Surg 2017;104:723-4. [Crossref] [PubMed]
- Terry PB, Traystman RJ, Newball HH, et al. Collateral ventilation in man. N Engl J Med 1978;298:10-5. [Crossref] [PubMed]
- Chahla M, Larson CD, Parekh KR, et al. Transpleural Ventilation via Spiracles in Severe Emphysema Increases Alveolar Ventilation. Chest 2016;149:e161-7. [Crossref] [PubMed]
- Eberlein M, Larson CD, Parekh KR, et al. Complete Transpleural Exhalation during a Bilateral Lung Transplant Operation. Ann Am Thorac Soc 2015;12:948-51. [Crossref] [PubMed]
- Khauli S, Bolukbas S, Reed RM, et al. Interlobar collateral ventilation in severe emphysema. Thorax 2016;71:1168-9. [Crossref] [PubMed]
- Khauli S, Abston E, Sajjad H, et al. Incomplete fissures are associated with increased alveolar ventilation via spiracles in severe emphysema. Interact Cardiovasc Thorac Surg 2017;25:851-5. [Crossref] [PubMed]
- Macchiarini P, Wain J, Almy S, et al. Experimental and clinical evaluation of a new synthetic, absorbable sealant to reduce air leaks in thoracic operations. J Thorac Cardiovasc Surg 1999;117:751-8. [Crossref] [PubMed]
- Yang HC, Chang HY. Novel air leak test using surfactant for lung surgery. J Thorac Dis 2018;10:6472-4. [Crossref] [PubMed]
- Kawai H. Problems with using the air leak test with Yang’s bubble solution during video-assisted thoracic surgery. J Thorac Dis 2019;11:630-1. [Crossref] [PubMed]
- Kim WH, Lee HC, Ryu HG, et al. Intraoperative ventilatory leak predicts prolonged air leak after lung resection: A retrospective observational study. PLoS One 2017;12:e0187598. [Crossref] [PubMed]
- Brunelli A, Salati M, Pompili C, et al. Intraoperative air leak measured after lobectomy is associated with postoperative duration of air leak. Eur J Cardiothorac Surg 2017;52:963-8. [Crossref] [PubMed]
- Zaraca F, Vaccarili M, Zaccagna G, et al. Can a standardised Ventilation Mechanical Test for quantitative intraoperative air leak grading reduce the length of hospital stay after video-assisted thoracoscopic surgery lobectomy? J Vis Surg 2017;3:179. [Crossref] [PubMed]


