
Changes in store for early-stage non-small cell lung cancer
Introduction
There has been dramatic progress with the introduction of targeted therapies and immune checkpoint inhibitors in the management of advanced non-small cell lung cancer (NSCLC). Biomarker-driven targeted therapy has revolutionized the management of oncogene-driven lung adenocarcinomas. Multiple generations of agents are now available for the treatment of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and ROS-aberrant lung adenocarcinomas. Furthermore, effective agents are either available or rapidly-emerging for BRAF/RET/MET/NTRK/ErbB2-positive subsets. In addition, immune biomarkers such as programmed death-ligand 1 (PD-L1) inform clinicians as to proper choices of immunotherapies, alone or in combination with chemotherapy, as treatment for the large majority of patients without actionable alterations. In fact, by now there is very little justification for the use of conventional chemotherapy alone as first-line therapy. We have similarly witnessed a major sea-change in the management of locally-advanced disease. The PACIFIC study has established a new standard with adjuvant durvalumab (Imfinzi) following concurrent chemoradiation leading to major survival benefits (1).
In this context, it is extremely disappointing that for the early-stage patients for whom cure is the most reachable, and for whom the impact of new systemic therapies could be the most substantial, little—if anything—has changed since the acceptance of adjuvant chemotherapy for resected NSCLC about two decades ago. While there have been advances in the treatment of early stage lung cancer through the use of stereotactic radiotherapy (2), robotic surgical techniques (3), and other interventions to avoid post-resection pulmonary complications (4), there have been few novel systemic therapies to offer. Instead, we have witnessed failed attempts at improving on the status quo, with negative results as to postoperative radiation (PORT meta-analysis) (5), anti-angiogenic (ECOG 1505) (6), and vaccine therapies (MAGRIT) (7). Furthermore, outside of nodal involvement and tumor size, no validated biomarkers exist in this setting to guide patient management. Adjuvant chemotherapy remains the standard of care for patients with resected NSCLC. The landmark International Adjuvant Lung Cancer Trial (IALT) showed that cisplatin-based postoperative chemotherapy improved survival, marking a new era in NSCLC management (8). The Lung Adjuvant Cisplatin Evaluation (LACE) meta-analysis of the key five trials of this era echoed these results, showing an overall survival benefit of 5.4% at five years (though adjuvant chemotherapy was found to be harmful in stage IA NSCLC) (9). Nonetheless, outcomes remain poor; the modest benefit offered by adjuvant chemotherapy indeed seems to lessen over time due to significant toxicities and other long-term complications of treatment. These complications are of particular concern in the elderly population of NSCLC patients, for whom the limited data suggest a survival benefit with chemotherapy, but at the expense of greater toxicity relative to their younger counterparts (10). The time is thus ripe to re-energize research with a focus on improving our curative strategies in this setting.
Mixed results from tyrosine kinase inhibitors (TKIs)
The introduction of targeted therapies in the adjuvant setting highlights this general disappointment. Over the past 15 years, EGFR TKI therapies have been tested in several adjuvant studies, yielding mixed results—partly due to misguided patient selection and partly due to poor trial design (Table 1). The non-molecularly selected NCIC CTG BR19 trial found no improvement in survival for patients receiving gefitinib compared to placebo in stage IB-IIIA surgically-resected patients, even in a subset analysis based on EGFR mutation status—although this subset was very small, leading to underpowered analyses (11). The RADIANT study randomized resected stage IB-IIIA IHC/FISH EGFR-positive patients to receive erlotinib or placebo following receipt of optional adjuvant chemotherapy, and similarly did not find any difference in disease-free or overall survival—not surprisingly, in retrospect, given incorrect biomarker choice (12). While a subset of patients with deletion 19 or L858R EGFR mutations in fact showed a disease-free survival advantage, the results were not significant owing to the hierarchical study design, and overall survival appeared identical. The subsequent multicenter phase II SELECT study of erlotinib for two years following resection of stage IB-IIIA EGFR-mutated NSCLC used more scientifically-valid biomarker selection and included only patients with EGFR mutations (13). The study certainly demonstrated excellent overall results and reached its endpoint of improved disease-free survival (DFS) as compared to historical controls, but the results are difficult to interpret in the absence of a true control group. Furthermore, a significant relapse rate soon after stopping adjuvant therapy is worrisome. This highlights the specific concern that adjuvant targeted therapy might lack actual curative effect, possibly leading only to delays in recurrence. The results, therefore, are not viewed as practice-changing. Yet another recent study, the phase II EVAN trial, showed improved disease-free survival (2 years) in stage IIIA patients receiving adjuvant erlotinib alone versus chemotherapy (14). The latest major trial to conclude, ADJUVANT, a randomized phase 3 Chinese study comparing adjuvant chemotherapy with gefitinib, did reach its primary endpoint of significant DFS improvement (median DFS of 28.7 vs. 18 months) (15). However, available overall survival (OS) data argue against a substantial true benefit, and the study is criticized for not offering standard of care chemotherapy in the gefitinib arm. Based on these studies, adjuvant EGFR TKIs may be beneficial to delaying recurrence, but clear benefits as to overall survival—the key endpoint in such studies—remain elusive.
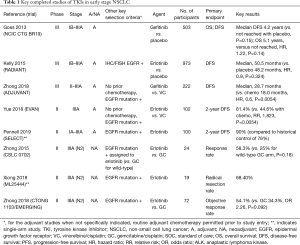
Full table
Erlotinib has also been studied in the neoadjuvant setting. The phase II study (CSLC 0702) by Zhong et al. demonstrated the feasibility of administering neoadjuvant erlotinib, stratified by EGFR mutation status, and showed a higher response rate in the EGFR+ erlotinib arm (16). Xiong et al. (ML25444) examined erlotinib’s role in achieving operability and demonstrated a radical resection rate of 68.4% in their sample (17). Most importantly, the multicenter phase II EMERGING study demonstrated a significant increase in progression-free survival (PFS) in the erlotinib arm (median PFS 21.5 vs. 11.9 months), although it did not reach statistical significance in its primary endpoint of objective response rate (18) Although OS data are immature and will warrant review to ascertain whether the survival benefit is durable, this study supports continued investigation of erlotinib and other TKIs in the neoadjuvant setting for early-stage EGFR+ NSCLC.
There is a continued effort to demonstrate the value of TKIs for early-stage NSCLC (Table 2), in the United States principally through the ALCHEMIST randomized controlled trials originally designed to test adjuvant targeted therapies (erlotinib for EGFR mutation, crizotinib for ALK rearrangement) versus placebo in resected stage IB-IIIA patients following receipt of adjuvant chemotherapy (19). Despite high hopes for ALCHEMIST, accrual has been slow, leading to concerns as to whether it can be successfully completed and—of more concern—whether it is becoming obsolete as more effective EGFR- and ALK-targeted agents have by now shown great success in the advanced setting. The potential of another first-generation TKI, icotinib, as adjuvant therapy for stage II-IIIA NSCLC is currently being investigated in a number of phase III studies in China, including ICWIP (NCT02125240), EVIDENCE (NCT02448797), and ICTAN (NCT01996098).
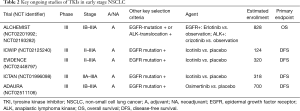
Full table
The important ADAURA study (NCT02511106) being conducted in a similar setting utilizes adjuvant osimertinib, a third-generation EGFR TKI with greater CNS penetrance that targets the T790M resistance mutation. Osimertinib has become the standard of care frontline option in advanced disease, thereby making ADAURA the most relevant of ongoing targeted adjuvant trials. In general, the lack of investment in adjuvant studies clearly has led to a series of missed opportunities over the last decade and a half, hopefully informing us now with regard to the next wave of studies ahead.
Immunotherapy in early stage NSCLC
Immunotherapy represents the next frontier of oncology, with checkpoint inhibitors already approved for a growing variety of cancer types by the United States Food and Drug Administration, including two anti-PD-1 and two anti-PD-L1 agents for the management of advanced and locally-advanced NSCLC (20). While checkpoint inhibitors are currently in widespread use for stage III/IV NSCLC, they remain investigational for early stages when their efficacy would likely be the most robust, and when their impact could be the most significant. Clearly, great efforts urgently need to be expanded to assess these agents. To this end, a variety of studies are ongoing in both the adjuvant and neoadjuvant settings (Tables 3,4).
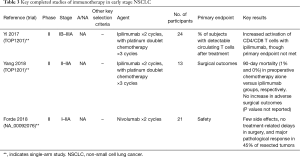
Full table
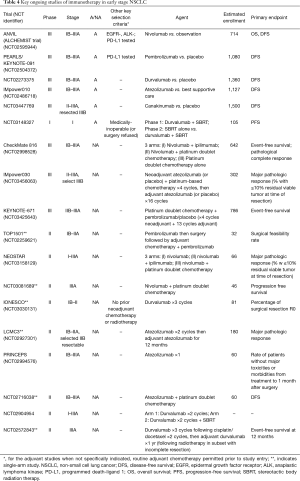
Full table
The administration of immunotherapy prior to surgical resection might be particularly advantageous, as larger tumors are thought to generate greater immune activity than micro-metastatic disease. Furthermore, the pathologic specimen allows for an early and robust readout of therapeutic activity, which might be particularly helpful in the selection of combination regimens for further studies. Completed efforts investigating this theory include TOP1201 and the pilot study by Forde and colleagues. The phase II TOP1201 study by Yi et al. [2017] demonstrated significant activation of CD4/CD8 lymphocytes after chemotherapy and ipilimumab in stage II-IIIA NSCLC, though it did not meet the primary endpoint of detecting a significant increase in circulating T cells with specificities against tumor-associated antigens (21). Yang et al. [2018] subsequently demonstrated the safety and feasibility of surgical resection in this study population (22). Forde and colleagues treated a cohort of 22 patients with resectable stage I-IIA NSCLC with 2 cycles of nivolumab prior to surgical resection (23). Neoadjuvant nivolumab was not only safe and feasible but was also associated with a 45% pathologic response rate in 9 of 20 patients who underwent resection, and a complete response in 3 patients. In addition, biomarker analysis of this cohort yielded remarkable data as to neoantigen discovery and identification of activated T cell clones, providing an excellent platform for future studies.
Trials in the neoadjuvant setting
The single arm nature of the above pilot studies nonetheless limits further interpretation of the data. While there are many phase II studies actively investigating neoadjuvant immunotherapy, the most notable include a series of now pivotal and potentially practice-changing phase III trials. IMpower030 (NCT03456063) is studying the role of neoadjuvant atezolizumab in resectable II, IIIA, or select IIIB NSCLC. KEYNOTE-671 (PEARLS, NCT03425643) is investigating the combination of neoadjuvant doublet chemotherapy with neoadjuvant/adjuvant administration of pembrolizumab versus placebo in patients with resectable stage IIB or IIIA NSCLC. Finally, CheckMate 816 (NCT02998528) is studying whether either combined nivolumab and ipilimumab, or nivolumab plus platinum doublet chemotherapy, is superior to doublet chemotherapy alone in the neoadjuvant treatment of stage IB–IIIA NSCLC.
Another immune checkpoint inhibitor, durvalumab, was recently approved for treatment of unresectable stage III NSCLC following the findings of the PACIFIC trial. This agent is now being investigated in a number of phase II neoadjuvant studies, including IONESCO (NCT03030131), NCT02572843, and NCT02904954. In addition to studying durvalumab therapy, NCT02904954 also examines whether there is an added benefit from the administration of stereotactic body radiation therapy (SBRT) prior to, or concurrently with, durvalumab therapy. Another ongoing trial, NCT03148327, examines this combination as adjuvant therapy in patients who are medically inoperable or who decline surgery. The investigation of SBRT in conjunction with durvalumab could further expand treatment options for early-stage patients who are undergoing immunotherapy thanks to the phenomenon known as the abscopal effect, through which local irradiation of the primary tumor is followed by regression of disease at non-irradiated metastatic sites—a phenomenon hypothesized to occur due to generation of a systemic immune response following the release of neoantigens from the irradiated tumor (24). Within the past decade, as the use of immunotherapy has been on the rise, there has been a growing consensus that a combination of radiotherapy and immunotherapy is superior to either modality alone, which certainly warrants further investigation in earlier-stage NSCLC. With the expanding use of curative radiation-based strategies in lung cancer management, these studies will provide key data for large groups of patients.
Trials in the Adjuvant Setting Studies are also ongoing into the use of immunotherapy in the adjuvant setting. The pivotal ANVIL trial (NCT02595944) is a component of the phase III ALCHEMIST trial studying the effects of adjuvant nivolumab versus observation for EGFR/ALK-negative patients, while the KEYNOTE-091 trial (NCT02504372) is investigating the adjuvant use of pembrolizumab versus placebo, with or without standard adjuvant chemotherapy, in resected NSCLC. Additional key adjuvant studies currently recruiting participants with stage IB-IIIA NSCLC are NCT02273375, investigating durvalumab versus placebo, and IMpower010 (NCT02486718), comparing adjuvant atezolizumab to best supportive care following 4 cycles of doublet chemotherapy. Another study currently in the recruitment phase, NCT03447769, aims to study the role of canakinumab as adjuvant therapy in patients with stage II-IIIA and completely-resected stage IIIB NSCLC. Canakinumab, a monoclonal antibody against the pro-inflammatory cytokine IL-1β, was originally approved for treatment of a spectrum of autoinflammatory conditions. Analysis of the results of the 2017 CANTOS cardiovascular study incidentally revealed a highly-significant reduction in lung cancer incidence and mortality in the canakinumab group relative to placebo, prompting further interest in its potential as a lung cancer therapy and opening novel avenues into the exploration of the role of the myeloid cell compartment in the tumor microenvironment (25).
Looking for better biomarkers
As the role of targeted therapies and immunotherapy in early-stage lung cancer continues to be explored, it is crucial that further investments be made in the development of better biomarkers to facilitate patient selection and risk stratification. Research continues into additional biomarkers beyond EGFR and ALK that either represent actionable therapeutic targets, or that are associated with risk of progression of early-stage NSCLC. For instance, the myPlan Lung Cancer proprietary prognostic test developed by Myriad Genetics, Inc. measures cell cycle progression genes; it has been validated in a cohort of 650 stage I-II NSCLC patients and found to be a more significant indicator of mortality than pathologic cancer stage (26). Another important biomarker being explored is tumor mutational burden (TMB), a measure of the number of mutations in a given tumor, and a surrogate marker for a tumor’s potential to generate an immune response to specific neoantigens. Initial studies in NSCLC have demonstrated an association between higher TMB and better response to immunotherapy; it appears that TMB might serve as a complimentary biomarker to PD-L1 IHC in patient selection (27). Further studies will be necessary in the setting of early-stage NSCLC as this biomarker is further elucidated and refined. Finally, plasma-derived circulating tumor DNA (ctDNA) holds the potential to further expand the role of personalized therapy—making it available to those patients not amenable to tissue biopsy and enabling non-invasive repeat sampling so that therapy can be modified as needed over the course of treatment, as the molecular profile of an individual’s cancer changes (28). Its most promising role in the definitive setting might be as a biomarker of residual microscopic disease, allowing patient enrichment in adjuvant studies following delivery of definitive surgery or radiation-based therapy. Indeed, the recently published study by Chaudhuri et al. highlights the potential tremendous utility of ctDNA testing for risk stratification in this context (29). Among the outcome measures of the ALCHEMIST Screening Trial (NCT02194738) are the identification of new mutations at time of cancer recurrence, and the correlation of ctDNA level with survival measures in these patients. Another study that has recently started recruitment (NCT03465241) is investigating the role of ctDNA dynamic monitoring of stage II-IIIA NSCLC to verify its prognostic/predictive effect.
Better data are emerging
In summary, following two decades of significant lull in this critical area of research, we are now seeing much better-designed neoadjuvant and adjuvant studies based on proper biomarker selection, and optimized treatment choices founded upon recent advances in the metastatic setting. As a result, there is real hope that in the coming years we will indeed see tremendous changes in the management of early-stage lung cancer. The only way this can be achieved is through support by all relevant thoracic disciplines to allow timely completion of important studies whose results could hold the key to improving cure rates for the large number of patients diagnosed with early-stage lung cancer worldwide.
Acknowledgments
None.
Footnote
Conflicts of Interest: Dr. B Halmos is receiving clinical research support from Merck, BMS, AbbVie, Novartis, GSK, Mirati, Boehringer-Ingelheim, Pfizer, Astra-Zeneca, Takeda and Guardant Health and has received consulting fees from Astra-Zeneca, Pfizer, Novartis, Guardant Health, Foundation Medicine, Merck, BMS, Genentech, Spectrum, Ignyta, Takeda and Boehringer-Ingelheim. The other authors have no conflicts of interest to declare.
References
- Antonia SJ, Villegas A, Daniel D, et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N Engl J Med 2018;379:2342-50. [Crossref] [PubMed]
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol 2015;16:630-7. [Crossref] [PubMed]
- Nakamura H. Systematic review of published studies on safety and efficacy of thoracoscopic and robot-assisted lobectomy for lung cancer. Ann Thorac Cardiovasc Surg 2014;20:93-8. [Crossref] [PubMed]
- Villeneuve PJ. Interventions to avoid pulmonary complications after lung cancer resection. J Thorac Dis 2018;10:S3781-S3788. [Crossref] [PubMed]
- Sakib N, Li N, Zhu X, et al. Effect of postoperative radiotherapy on outcome in resectable stage IIA-N2 non-small-cell lung cancer: an updated meta-analysis. Nucl Med Commun 2018;39:51-9. [Crossref] [PubMed]
- Wakelee HA, Dahlberg SE, Keller SM, et al. Adjuvant chemotherapy with or without bevacizumab in patients with resected non-small-cell lung cancer (E1505): an open-label, multicenter, randomized, phase 3 trial. Lancet Oncol 2017;18:1610-23. [Crossref] [PubMed]
- Vansteenkiste JF, Cho BC, Vanakesa T, et al. Efficacy of the MAGE-A3 cancer immunotherapeutic as adjuvant therapy in patients with resected MAGE-A3-positive non-small-cell lung cancer (MAGRIT): a randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2016;17:822-35. [Crossref] [PubMed]
- Arriagada R, Bergman B, Dunant A, et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med 2004;350:351-60. [Crossref] [PubMed]
- Pignon JP, Tribodet H, Scagliotti GV, et al. Lung adjuvant cisplatin evaluation: a pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552-9. [Crossref] [PubMed]
- Veluswamy RR, Levy B, Wisnivesky JP. Chemotherapy in elderly patients with nonsmall cell lung cancer. Curr Opin Pulm Med 2016;22:336-43. [Crossref] [PubMed]
- Goss GD, O’Callaghan C, Lorimer I, et al. Gefitinib versus placebo in completely resected non-small-cell lung cancer: results of the NCIC CTG BR19 study. J Clin Oncol 2013;31:3320-6. [Crossref] [PubMed]
- Kelly K, Altorki NK, Eberhardt WE, et al. Adjuvant erlotinib versus placebo in patients with stage IB-IIIA non-small-cell lung cancer (RADIANT): a randomized, double-blind, phase III trial. J Clin Oncol 2015;33:4007-14. [Crossref] [PubMed]
- Pennell NA, Neal JW, Chaft JE, et al. SELECT: a phase II trial of adjuvant erlotinib in patients with resected epidermal growth factor receptor-mutant non-small-cell lung cancer. J Clin Oncol 2019;37:97-104. [Crossref] [PubMed]
- Yue D, Xu S, Wang Q, et al. Erlotinib versus vinorelbine plus cisplatin as adjuvant therapy in Chinese patients with stage IIIA EGFR mutation-positive non-small-cell lung cancer (EVAN): a randomised, open-label, phase 2 trial. Lancet Respir Med 2018;6:863-73. [Crossref] [PubMed]
- Zhong WZ, Wang Q, Mao WM, et al. Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II-IIIA (N1-N2) EGFR-mutant NSCLC (ADJUVANT/CTONG1104): a randomised, open-label, phase 3 study. Lancet Oncol 2018;19:139-48. [Crossref] [PubMed]
- Zhong W, Yang X, Yan H, et al. Phase II study of biomarker-guided neoadjuvant treatment strategy for IIIA-N2 non-small cell lung cancer based on epidermal growth factor receptor mutation status. J Hematol Oncol 2015;8:54. [Crossref] [PubMed]
- Xiong L, Li R, Sun J, et al. Erlotinib as Neoadjuvant Therapy in Stage IIIA (N2) EGFR Mutation-Positive Non-Small Cell Lung Cancer: A Prospective Single-Arm, Phase II Study. Oncologist 2019;24:157-e64. [Crossref] [PubMed]
- Zhong WZ, Wu YL, Chen KN, et al. Erlotinib versus gemcitabine plus cisplatin as neo-adjuvant treatment for stage IIIA-N2 EGFR-mutation non-small cell lung cancer (EMERGING): A randomised study. Ann Oncol 2018.29.
- Govindan R, Mandrekar SJ, Gerber DE, et al. ALCHEMIST trials: a golden opportunity to transform outcomes in early-stage non-small-cell lung cancer. Clin Cancer Res 2015;21:5439-44. [Crossref] [PubMed]
- Morgensztern D, Herbst RS. Nivolumab and pembrolizumab for non-small cell lung cancer. Clin Cancer Res 2016;22:3713-7. [Crossref] [PubMed]
- Yi JS, Ready N, Healy P, et al. Immune activation in early-stage non-small cell lung cancer patients receiving neoadjuvant chemotherapy plus ipilimumab. Clin Cancer Res 2017;23:7474-82. [Crossref] [PubMed]
- Yang CJ, McSherry F, Mayne NR, et al. Surgical outcomes after neoadjuvant chemotherapy and ipilimumab for non-small cell lung cancer. Ann Thorac Surg 2018;105:924-9. [Crossref] [PubMed]
- Forde PM, Chaft JE, Smith KN, et al. Neoadjuvant PD-1 blockade in resectable lung cancer. N Engl J Med 2018;378:1976-86. [Crossref] [PubMed]
- Cushman TR, Gomez D, Kumar R, et al. Combining radiation plus immunotherapy to improve systemic immune response. J Thorac Dis 2018;10:S468-S479. [Crossref] [PubMed]
- Ridker PM, Everett BM, Thuren T, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119-31. [Crossref] [PubMed]
- Zheng Y, Bueno R. Commercially available prognostic molecular models in early-stage lung cancer: a review of the Pervenio Lung RS and Myriad myPlan Lung Cancer tests. Expert Rev Mol Diagn 2015;15:589-96. [Crossref] [PubMed]
- Goodman AM, Kato S, Bazhenova L, et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol Cancer Ther 2017;16:2598-608. [Crossref] [PubMed]
- Abbosh C, Birbak NJ, Swanton C. Early stage NSCLC – challenges to implementing ctDNA-based screening and MRD detection. Nat Rev Clin Oncol 2018;15:577-86. [Crossref] [PubMed]
- Chaudhuri AA, Chabon JJ, Lovejoy AF, et al. Early detection of molecular residual disease in localized lung cancer by circulating tumor DNA profiling. Cancer Discov 2017;7:1394-403. [Crossref] [PubMed]


