
VEGFR endocytosis regulates the angiogenesis in a mouse model of hindlimb ischemia
Introduction
Atherosclerosis is the leading cause of various diseases of the cardiovascular system, such as coronary artery diseases and peripheral artery diseases (PAD), which most commonly affect arteries of lower limbs (1). The most critical ischemia, which leads to permanent disability, has been more than 500 cases per million population per year all over the world. During the process of ischemia, the organ tissue will spontaneously develop collateral circulation in response to the signals of hypoxia (2,3). The formation of collateral circulation post ischemia contains neovascularization and angiogenesis, which is also the basis of the treatment approaches (4).
The current main therapies concerning atherosclerotic diseases such as PAD comprises of both interventional and surgical revascularization, nonetheless the latest seemingly more promising therapy is injecting angiogenetic agents analogous to vascular endothelial growth factor (VEGF) or using stem/progenitor cell-based therapy. Many researches are focused on discovering sources of highly multi-potent stem cells and methods of clinical application. Endothelial progenitor cells (EPCs), which are mainly responsible for angiogenesis in ischemic environment, are one of the cell-based therapies for promoting neovascularization in PAD. Several sources of EPCs have been discovered in recent years, other cell types such as adipose tissue derived regenerative cells and induced pluripotent stem cells are also a scientific breakthrough for establishing a potential cell source to be used in the treatment of ischemic cardiovascular diseases. Many different cell types have shown promising outcomes in clinical trials (5). Although cell-based therapy for PAD appears promising, such questions as optimal cell source and appropriate delivery cell numbers still remain in order to promote the efficacy of stem cell therapy and enhance the angiogenesis post the ischemia (6).
Angiogenesis is a spatiotemporal process with crucial points during development, growth, and wound healing in adult organisms (7). The VEGF families and their receptors, VEGFR1-2, have been proven to be key regulators of normal and pathological angiogenesis. Most researches agree that VEGF functions by binding with VEGFR and triggering the downstream signaling pathways and thus starting the angiogenesis process (8-10). Recent studies found out that VEGFR endocytosis could actually regulate the angiogenesis by protecting VEGFR against plasma membrane cleavage. Nakayama et al. (11) revealed that during the development of retina, vessel growth was dynamically controlled through spatial control of VEGFR endocytosis. With its effect on the regulation of angiogenesis, VEGFR endocytosis has shown great potential in the investigation of proangiogenic therapies (12,13).
Our team previously reported that promoting VEGFR endocytosis would enhance angiogenesis independent of the VEGF expression. We also showed in vitro that atypical protein kinase C (αPKC) inhibitor could enhance the VEGFR endocytosis and the formation of vascular networks (14). However currently there is no investigation on the effect of VEGFR endocytosis in the disease model of ischemic disorders, such as PAD and myocardial infarction. Therefore, we intended to investigate the effect of VEGFR endocytosis on tissue ischemia in vivo by using the mouse hindlimb ischemia model in this study. We hypothesized that promoting VEGFR endocytosis could be a feasible strategy to improve the efficacy of current therapies towards ameliorating hindlimb ischemia.
Methods
Analysis of VEGFR endocytosis after treated with αPKCi and dynasore by western blotting, immunostaining and confocal microscopy
Preparation of EPCs
The isolation, culture and characterization of EPCs were successfully implemented in our group and described in detail in Journal of Molecular and Cellular Biochemistry (15) and International Heart Journal (14).
Western blotting
Western blotting was performed as previously reported. Briefly, the total proteins were extracted from EPCs and EPCs treated with αPKC inhibitor (5 µM, Calbiochem, San Diego, USA) for 30 min, and Dynasore (100 µM, Sigma-Aldrich, Shanghai, China) for 2 hours, while the membrane proteins were extracted by the Membrane and cytosol protein extraction kit (Beyotime). The primary antibody was rabbit anti-VEGF receptor 2 antibody (1:1,000, ab39638, Abcam), followed by a peroxidase-conjugated secondary antibody. GAPDH was used as an internal control.
Immunostaining
EPCs were seeded at a density of 3×105 in 6 cm dishes and incubated for 24 h. Then the cells were treated with αPKCi for 30 min, and dynasore for 2 hours. After been washed with PBS and fixed in 4% paraformaldehyde for 15 minutes, the cells were treated with 0.2% Triton X-100 for 2 minutes. Five percent bovine serum albumin were used to block for 30 minutes. The cells were then incubated in the primary antibody against VEGFR2 (1:100, 55B11, Cell Signaling Technology, Beverly, MA, USA) at 4 °C overnight. Then washed the cells with PBS and incubated with a FITC-conjugated anti-rabbit secondary antibody (1:1,000, Invitrogen, Carlsbad, CA, USA) for 1 hour at 37 °C. Washed the cells once again with PBS and counterstaining with DAPI. The cell samples were observed under a confocal microscope (Zeiss, Munich, Germany).
Analysis of angiogenesis in vitro after treated with αPKCi and dynasore by Matrigel matrix
EPCs were seeded on the top of 80 µL pre-polymerization growth factor-reduced Matrigel (BD Biosciences, UK) in a 96-well plate (1.5×104 cells per well) in a humidified incubator at 37 °C for 8 hours. Then the floating cells were removed and the endothelial networks were checked under microscope. The length of cords and the number of junctions formed were also assessed using the Bioquant Image Analysis System (R&M Biometrics).
Mouse hindlimb ischemia model
Male mice aged 10–12 weeks with immune-deficiency (n=24) were conducted an intraperitoneal injection with ketamine hydrochloride (dose for 90 mg/kg) for anaesthesia. All of the mice’s left femoral artery were separated from the femoral nerve and vein, ligated, and excised to induce ischemia. Then all mice were randomly divided into four groups (n=6 each): sham-treated (PBS) group, EPCs group (4×106 cells per mouse), EPCs + αPKCi (100 µM, Calbiochem, San Diego, USA) group and EPCs + dynasore (100 µM, Sigma-Aldrich, Shanghai, China) group. Cells or equal volume PBS were injected at two different sites (4×106 cells; 50 uL per site) on thigh muscles of ischemic limbs 1 minute after the operation. As previously described, the EPCs were pre-treated with αPKCi/dynasore before injection.
Hindlimb blood flow measurement
Hindlimb blood flow was measured by the imaging device with laser Doppler perfusion imaging on days 0, 7, 14 and 28 after the operation. Mice were anesthetized and placed on a 37 °C heating plate for 5 minutes. Blood flow was measured from scanning images, and the perfusion ratio of ischemic limbs were quantified by averaging relative units of flux from the knee to the toe compared with non-ischemic limbs (PIMsoft Software by Perimed Med, Sweden).
Analysis of angiogenesis in vivo by immunohistochemistry
On the day 28 after operation, the quadriceps femoris muscles of all the mice were harvested and prepared for immunohistochemical staining to assess the blood vessel density and area. All of the specimens were fixed in formaldehyde, dehydrated in a graded ethanol series, and embedded in paraffin. Paraffin embedded specimens were sliced into 4-µm sections, some of the tissue sections were stained with hematoxylin and eosin. The presence of collateral vessels was assessed by immunohistochemical staining with rabbit anti-alpha-smooth muscle actin (α-SMA) (Abcam Cambridge, MA, USA) and rabbit anti-CD31 antibody (Abcam Cambridge, MA, USA). For quantification of capillary density, eight images per slide from individual samples (three slides per sample) of each group were analyzed.
Tracking the injected EPCs with eGFP labeling
Before the transplantation of EPCs, the cells were transfected with enhanced green fluorescent protein (eGFP) by lentivirus. Then 4×106 cells were injected into the ischemic area per mice. The quadriceps femoris muscle of ischemic limb were harvested 7 days after the injection of cells and embedded with optimal cutting temperature (OCT) compound when finished the sucrose gradient dehydration. The tissue blocks in the OCT were snapped frozen in liquid nitrogen and then sectioned at 10 µm. After counterstaining with DAPI, the sections were photographed with fluorescence confocal microscope.
Analysis of growth factors and cytokines in the peripheral blood
On day 28 after the operation, mice peripheral blood was collected and analyzed for growth factors and cytokines such as IL-6, GM-CSF and VEGF. The concentrations of these growth factors were measured by the ELISA kits followed by the manufacturer’s instructions. Each sample was run in duplicate. Growth factor and cytokine concentrations were determined from standard curves and expressed as pg/mL or ng/mL.
Statistical analysis
All values are shown as the means ± SEM. Comparisons between two groups were used by Student’s t-test, while in more than two groups one-way ANOVA followed by Bonferroni’s post-hoc test were used. P value <0.05 was considered statistical significance.
The protocol was approved by Zhongshan Hospital Institutional Review Board (No. 2007-34).
Results
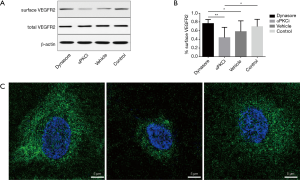
VEGFR internalization after treating the EPCs with αPKCi, dynasore and vehicle (0.1% DMSO)
From Figure 1A and 1B, after treating the EPCs with αPKCi and dynasore, the total VEGFR2 expression in EPCs did not altered. The surface VEGFR2 expression in αPKCi group decreased with statistical significance compared to the dynasore/and control group, which implied an increase in VEGFR endocytosis. The immunostaining confocal imaging showed the ligand-receptor complexes accumulated more in the perinuclear region of αPKCi treated EPCs but were more restricted to the peripheral compartments of EPCs with the treatment of dynasore (Figure 1C).
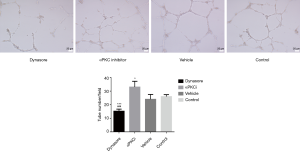
Effects of αPKCi or dynasore on EPCs angiogenesis in vitro by Matrigel assay
The result of Matrigel matrix angiogenesis assay showed that EPCs treated with dynasore experienced a decrease in angiogenesis compared with αPKCi and control group (Figure 2).
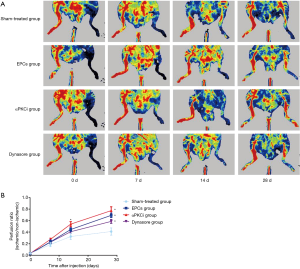
Blood flow measurement after injection of EPCs treated with αPKCi and dynasore
The blood flow measurement of hindlimb was compared between different groups. For the whole period the ischemic hindlimb blood flow was less than the contralateral hindlimb among all the groups. From postoperative day 14, the blood flow of the ischemic hindlimb in the αPKCi group was the highest among all the four groups. Although ischemic limb blood flow was higher with EPCs than sham-treated group on postoperative day 28, it was far higher in the αPKCi group, which implied that increasing endocytosis would improve the ischemic limb blood perfusion (Figure 3). In dynasore group, ischemic limb blood perfusion had the similar blood perfusion compared with EPCs group (Figure 3).
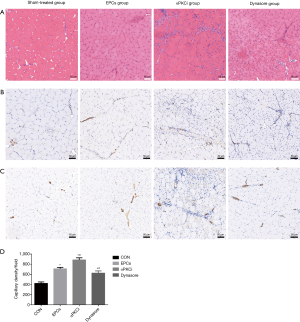
Angiogenesis in vivo by immunohistochemistry (IHC) analysis
The degree of angiogenesis in vivo was analysed by IHC and assessment of α-SMA-positive vessels, and CD31-positive blood vessel density. In ischemic hindlimb muscles of different groups on postoperative days 28. First of all, HE staining of all the groups were showed (Figure 4A). CD31 is often used to evaluate the degree of angiogenesis. The EPCs group experienced an increase in the number of CD31-positive vessels in comparison to sham-treated group. In αPKCi group, there were a bigger proportion of CD31-postive vessels, which implied elevation of angiogenesis due to an increase of VEGFR endocytosis. In dynasore group, the CD31-positive vessels among ischemic limb tissues decreased compared with EPCs group. α-SMA are the actin isoform typical of smooth muscle cells in vascular walls, which can also indicate the level of angiogenesis. There was more presence of α-SMA-positive vessels in the EPCs group than in the sham-treated group (Figure 4C). In αPKCi group and dynasore group, the similar tendency could be seen (Figure 4C). The EPCs + αPKCi, EPCs + dynasore, and EPCs group showed an increase in capillary density compared to control group (Figure 4D).
Tracking of eGFP-labelled EPCs in vivo
In order to demonstrate whether the enhancement of angiogenesis is produced by increasing VEGFR endocytosis, we labeled EPCs with eGFP and thus tracked the injected cells. Treatment of αPKCi did not change the retention rate of injected EPCs (Figure 5). Treatment of αPKCi increased the VEGFR endocytosis as well as angiogenesis in vitro and in vivo (Figure 5).
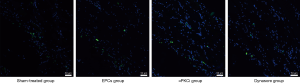
Taken together, these findings demonstrated that the regulation of VEGFR endocytosis allows the EPCs to enhance their angiogenic properties.
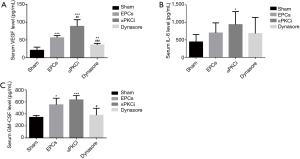
Peripheral blood growth factors and cytokines
Next, we tested the concentration of growth factor and cytokines by ELISA to identify the candidate proteins underscoring angiogenesis. At day 28 after surgery, up-regulated levels of VEGF, interleukin 6 and granulocyte-macrophage colony stimulating factor (GM-CSF) were shown in all of the three groups compared with sham-treated group. But compared with EPCs group, the level of VEGF in αPKCi treated group is much higher (Figure 6A, P<0.05). There was no significant difference of IL-6 level in the three group (Figure 6B). The level of GM-CSF in the dynasore group was lower than the EPCs group (Figure 6C, P<0.05).
Discussion
We demonstrated in this research that the regulation VEGFR endocytosis would be a favorable mechanism of regulating angiogenesis and revascularization in the mice model of hindlimb ischemia.
Nowadays growing numbers of researches are focused on the regulation on angiogenesis in many diseases, including tumors, myocardial infarction and peripheral arterial diseases. Recent studies revealed several potential factors involving the regulation of angiogenesis, and the role of VEGF families and VEGF receptors remain the most important portion. According to the traditional views, activation of VEGFR by VEGF induces phosphorylation, dimerization, endocytosis and a downstream signaling pathway which regulate endothelial cells migration, survival and proliferation (16-18). Interestingly, based on a research by Ewan et al., VEGFR2 could experience abundant endocytosis even in the absence of VEGF (19). The VEGFR endocytosis will trigger downstream cell signaling and produce various functions such as angiogenesis.
However, the biological function of VEGFR endocytosis remains unclear. According to Basagiannis et al. (12), VEGFR2 experienced constitutive endocytosis without ligand and recycling. The function of VEGFR2 endocytosis is to protect VEGFR against plasma membrane cleavage and preserve the functional portion of the receptor until the activation by VEGF. We previously discovered the effect of a decrease in EPCs angiogenesis caused by mechanical stress in vitro could be reversed by upregulating the membrane VEGFR endocytosis, which was independent of VEGF expression. In this research, we found out that through enhancing the VEGFR endocytosis of EPCs, the angiogenesis will increase both in vitro and in vivo. Hence, we assume that the potential function of VEGFR endocytosis might involve in promoting angiogenesis and neovascularization, which is similar to the study of Nakayama et al. (11).
As for the mechanism of angiogenesis in EPCs remains controversial (20,21). Currently there are two potential mechanisms. One is the direct function of EPCs incorporated into vessel walls. The other is by paracrine effect. Our research focused on the effect of promoting angiogenesis by enhancing VEGFR endocytosis signaling on the basis of the direct angiogenetic function of EPCs. In the meanwhile, we found out that αPKCi -intervened EPCs enhanced the paracrine effect in vivo, which suggested that angiogenic mechanism involves not only EPCs per se, but also induce endogenous growth factors and cytokines. Therefore, it helped reach our conclusion that through enhancing the VEGFR endocytosis of EPCs would regulating angiogenesis and revascularization in the mice model of hindlimb ischemia. We understand that there remain some problems in this cell-based therapy, such as a low engraftment rate, and therefore we hope that by strengthening the capacity of each implanted cell, these problems could be minimized. Our research at least provides a feasible method to enhance the capacity of implanted cells in order to improve the treatment outcomes. Overall, this research provides new insight into the cell therapy towards ischemic diseases such as PAD and myocardial infarction by enhancing VEGFR endocytosis independent of VEGF, which could subsequently increase the angiogenesis in the mice model of hindlimb ischemia.
Nevertheless, there are some limitations of this study. First, endocytosis is a complicated process which involves internalization of the ligand-receptor complexes and the mediation of the specificity, amplitude and duration of the intracellular signaling events (22-25). More detailed mechanism that VEGFR endocytosis involved in the angiogenesis needs to be studied. Basagiannis et al. showed the protective effect of VEGFR endocytosis by preventing VEGFR from plasma membrane cleavage. By inhibiting VEGFR endocytosis, Basagiannis et al. found increased production of s100 (a N-terminal soluble fragment of VEGFR2) in the extracellular space and p130 (a residual C-terminal part of VEGFR2) remaining at the plasma membrane. In further studies, we may focus on the s100 and p130 during the promotion and inhibition of VEGFR endocytosis (12,13). In addition, the VEGFR endocytosis is mediated by many pathways, including clathrin pathway, ubiquitination pathway and ephrin pathway (26-30). Hence to study the pathways of VEGFR endocytosis and compare their effects on angiogenesis would be another direction in further studies. Nonetheless, these limitations should not devalue the important implication that VEGFR endocytosis involved in the angiogenesis and vasculogenesis in model of PAD.
Conclusions
VEGFR endocytosis plays an important role in the angiogenesis of the ischemic hindlimb model. By using atypical PKC inhibitor, which increased the VEGFR endocytosis, the angiogenesis in the mice model was promoted. Therefore, the study shows that regulation of VEGFR endocytosis represents a valuable method of improving angiogenesis and thus revascularization in diseases such as PAD.
Acknowledgments
Funding: This work was supported by Chinese Cardiovascular Association CMVD Grant (2018-CCA-CMVD-03); Shanghai Health and Family Planning Commission (20164Y0036); Young Investigator Fund of Zhongshan Hospital, Fudan University (2016ZSQN05).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The protocol was approved by Zhongshan Hospital Institutional Review Board (No. 2007-34).
References
- Liu Q, Chen Z, Terry T, et al. Intra-arterial transplantation of adult bone marrow cells restores blood flow and regenerates skeletal muscle in ischemic limbs. Vasc Endovascular Surg 2009;43:433-43. [Crossref] [PubMed]
- Franz RW, Shah KJ, Johnson JD, et al. Short- to mid-term results using autologous bone-marrow mononuclear cell implantation therapy as a limb salvage procedure in patients with severe peripheral arterial disease. Vasc Endovascular Surg 2011;45:398-406. [Crossref] [PubMed]
- Mohler ER 3rd. Peripheral arterial disease: identification and implications. Arch Intern Med 2003;163:2306-14. [Crossref] [PubMed]
- Fujita Y, Kawamoto A. Stem cell-based peripheral vascular regeneration. Adv Drug Deliv Rev 2017;120:25-40. [Crossref] [PubMed]
- Gomez RA, Fernandez JD, Cabrera M, et al. Possible predictors of poor angiogenesis after hematopoietic stem cell autograft for lower limb ischemia. MEDICC Rev 2012;14:31-6. [PubMed]
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Eur J Vasc Endovasc Surg 2007;33 Suppl 1:S1-75. [Crossref] [PubMed]
- Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298-307. [Crossref] [PubMed]
- Koch S, Tugues S, Li X, et al. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011;437:169-83. [Crossref] [PubMed]
- Herbert SP, Stainier DY. Molecular control of endothelial cell behaviour during blood vessel morphogenesis. Nat Rev Mol Cell Biol 2011;12:551-64. [Crossref] [PubMed]
- Zhang Y, Bai Y, Jing Q, et al. Functions and Regeneration of Mature Cardiac Lymphatic Vessels in Atherosclerosis, Myocardial Infarction, and Heart Failure. Lymphat Res Biol 2018. [Epub ahead of print]. [Crossref] [PubMed]
- Nakayama M, Nakayama A, van Lessen M, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol 2013;15:249-60. [Crossref] [PubMed]
- Basagiannis D, Christoforidis S. Constitutive Endocytosis of VEGFR2 Protects the Receptor against Shedding. J Biol Chem 2016;291:16892-903. [Crossref] [PubMed]
- Basagiannis D, Zografou S, Galanopoulou K, et al. Dynasore impairs VEGFR2 signalling in an endocytosis-independent manner. Sci Rep 2017;7:45035. [Crossref] [PubMed]
- Bai Y, Wang X, Shen L, et al. Mechanical Stress Regulates Endothelial Progenitor Cell Angiogenesis Through VEGF Receptor Endocytosis. Int Heart J 2016;57:356-62. [Crossref] [PubMed]
- Shen L, Gao Y, Qian J, et al. The role of SDF-1alpha/Rac pathway in the regulation of endothelial progenitor cell polarity; homing and expression of Rac1, Rac2 during endothelial repair. Mol Cell Biochem 2012;365:1-7. [Crossref] [PubMed]
- Olsson AK, Dimberg A, Kreuger J, Claesson-Welsh L. VEGF receptor signalling-in control of vascular function. Nat Rev Mol Cell Biol 2006;7:359-71. [Crossref] [PubMed]
- Bruns AF, Herbert SP, Odell AF, et al. Ligand-stimulated VEGFR2 signaling is regulated by co-ordinated trafficking and proteolysis. Traffic 2010;11:161-74. [Crossref] [PubMed]
- Chen TT, Luque A, Lee S, et al. Anchorage of VEGF to the extracellular matrix conveys differential signaling responses to endothelial cells. J Cell Biol 2010;188:595-609. [Crossref] [PubMed]
- Ewan LC, Jopling HM, Jia H, et al. Intrinsic tyrosine kinase activity is required for vascular endothelial growth factor receptor 2 ubiquitination, sorting and degradation in endothelial cells. Traffic 2006;7:1270-82. [Crossref] [PubMed]
- Cooke JP, Losordo DW. Modulating the vascular response to limb ischemia: angiogenic and cell therapies. Circ Res 2015;116:1561-78. [Crossref] [PubMed]
- Yoder MC. Endothelial progenitor cell: a blood cell by many other names may serve similar functions. J Mol Med (Berl) 2013;91:285-95. [Crossref] [PubMed]
- Miaczynska M, Pelkmans L, Zerial M. Not just a sink: endosomes in control of signal transduction. Curr Opin Cell Biol 2004;16:400-6. [Crossref] [PubMed]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol 2009;10:609-22. [Crossref] [PubMed]
- Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol 2011;23:393-403. [Crossref] [PubMed]
- Irannejad R, Tsvetanova NG, Lobingier BT, et al. Effects of endocytosis on receptor-mediated signaling. Curr Opin Cell Biol 2015;35:137-43. [Crossref] [PubMed]
- McMahon HT, Boucrot E. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 2011;12:517-33. [Crossref] [PubMed]
- Meyer RD, Srinivasan S, Singh AJ, et al. PEST motif serine and tyrosine phosphorylation controls vascular endothelial growth factor receptor 2 stability and downregulation. Mol Cell Biol 2011;31:2010-25. [Crossref] [PubMed]
- Haglund K, Sigismund S, Polo S, et al. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol 2003;5:461-6. [Crossref] [PubMed]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell 1998;93:741-53. [Crossref] [PubMed]
- Goh LK, Huang F, Kim W, et al. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol. 2010;189:871-83. [Crossref] [PubMed]


