
Incidence and clinical relevance of non-small cell lung cancer lymph node micro-metastasis detected by staging endobronchial ultrasound-guided transbronchial needle aspiration
Introduction
Non-small cell lung cancer (NSCLC) remains a disproportionate cause of cancer mortality relative to its incidence (1). One of the many treatment challenges associated with NSCLC is the frequency of recurrence following definitive treatment of early stage disease (2-5).
Using immunohistochemistry (IHC) staining for pancytokeratin, lymph node (LN) micro-metastases (MMs) have been demonstrated in patients with NSCLC deemed to have negative LNs by conventional hematoxylin and eosin (H+E) staining. The presence of LN MM has long been associated with worsened outcomes in NSCLC patients (6). Subsequent work focused on detection of MM in NSCLC using IHC staining for Ber-Ep4/Ep-CAM, and MMs were detected in 15.2% to 31.2% of patients (7-9).
Recently, two large prospective trials reported detection of LN MMs in 22% and 14% of early stage NSCLC patients, and in both studies LN MMs had an association with worsened clinical outcomes (10,11). Micrometastases have been suggested as one possible explanation for the relatively poor outcomes of definitively surgically treated NSCLC patients (6-11).
Endobonchial ultrasound with transbronchial needle aspiration (EBUS-TBNA) or similar needle aspiration techniques are currently recommended as the first line invasive mediastinal staging modality in patients with a high incidence of N2 or N3 disease (12). In addition, many early stage NSCLC patients have comorbidities which preclude definitive surgery or are unwilling to undergo surgery (13,14). A substantial number of these patients are treated with stereotactic body radiotherapy (SBRT) or conventionally fractionated therapy as definitive treatment, procedures which do not directly involve detailed sampling of local LNs (15-17). Positron emission tomography (PET) may not be sufficient to adequately stage patients being considered for definitive radiation therapy and invasive mediastinal staging with EBUS may have a role in pre-radiation treatment planning (18,19). As such, EBUS-TBNA has a substantial role in the pre-treatment staging in a large proportion of lung cancer patients, even those with putative early stage disease.
The incidence of IHC-detectible LN MMs in patients with NSCLC as detected by EBUS-TBNA is unknown. Given the paucity of data in this realm and the first line role of EBUS-TBNA in mediastinal assessment in many clinical situations for NSCLC, we retrospectively evaluated the incidence of IHC detectable LN MMs in EBUS-TBNA specimens obtained for lung cancer staging purposes and correlated the results with clinical outcomes
Methods
Patient identification
All patients undergoing EBUS-TBNA for NSCLC LN staging at the University of North Carolina between September of 2013 and October 2017 were eligible for study inclusion. Staging indications at our institution include enlarged LNs on cross-sectional imaging, PET positivity of a LN, central tumor location (within 2/3 of the distance to the pleura from the mediastinum), or consideration for stereotactic body radiation therapy (SBRT) if the patient can tolerate a diagnostic procedure. These indications are slightly modified from the those recommended by the American College of Chest Physicians guidelines (12). Patients were identified using provider-maintained case lists, operating room or procedure room electronic schedules, and tumor board patient presentations. Defined blocks of 7.5 to 23 months were evaluated for each EBUS-TBNA provider (JA, MPR, JL, BH) during which time each EBUS-TBNA case performed was evaluated for inclusion. The blocks were selected based on convenience with respect to the availability of case lists from the EBUS-TBNA attending providers (JA, MPR, JL, BH). All patients undergoing a procedure during a defined block were evaluated. Patient identification continued until the funding for the study was exhausted. Patients presented to the lung cancer tumor board fitting study inclusion criteria while cases were being identified (Jan 2016 to Oct 2017) were also screened for inclusion.
Regardless of the method of identification, all identified patients were screened for study inclusion. Patients were required to have undergone an EBUS-TBNA procedure of at least one LN station for staging of suspected NSCLC. Tissue diagnosis confirming NSCLC was required within 3 months of performance of EBUS-TBNA. It was not required that the diagnosis of NSCLC be obtained after the EBUS-TBNA was performed (a few patients were included who had a CT-guided biopsy of a nodule which was positive for NSCLC and then went on for EBUS-TBNA staging). Patient wishes regarding treatment were not taken into account and thus patients who choose to forgo curative intent therapy were not excluded. Patients were excluded if no definitive tissue diagnosis of NSCLC was obtained within 3 months of initial EBUS-TBNA, if their EBUS-TBNA was positive for N2 or N3 disease by conventional H+E staining at any site, or if extrathoracic metastatic disease was present at the time of the EBUS-TBNA. Finally, patients were excluded if their index lung lesion was a squamous cell cancer and they had a concurrent extrathoracic primary squamous cell cancer and their lung lesion could not be reliably differentiated from a metastasis by immunohistochemical staining. A prior history of NSCLC was not exclusion criteria in patients without a concern for local recurrence. This study was approved by the University of North Carolina at Chapel Hill Program Review Committee and Institutional Review Board (LCCC 1425 and 14-1755, respectively).
Specimen acquisition and processing
All patients underwent computed tomography (CT) scanning prior to their EBUS-TBNA procedure. Positron emission tomography (PET) with CT was performed at the discretion of the referring or procedural team. All procedures were performed in an operating room setting under general anesthesia using a laryngeal mask airway or endotracheal tube at the discretion of the anesthesia team. Rapid on-site cytologic evaluation (ROSE) was available for all cases to determine the presence or absence of obvious LN metastasis. All patients underwent a complete airway inspection using a standard white light bronchoscope, BF-T180 (Olympus; Tokyo, Japan), to evaluate for occult endobronchial disease. EBUS-guided evaluation of the mediastinal and hilar LNs was performed in a systematic fashion starting with the N3 nodes and proceeding in a systematic fashion to N2 and N1 nodes. EBUS-TBNA was performed for LNs larger than 5 mm in shortest diameter using an EBUS bronchoscope, BFUC-180F (Olympus; Tokyo, Japan), and a dedicated 21- or 22-g EBUS needle (Olympus; Tokyo, Japan) (20).
Prior to study inclusion, cytologic specimens (slides and cell blocks) from the EBUS-TBNA procedure were evaluated by a cytopathologist using conventional staining according to standard guidelines. After selection for inclusion in this study, the smears and cell blocks from each TBNA specimen at each biopsied LN station were re-reviewed by a study pathologist (JH). Samples containing only blood were excluded from further review. Cell blocks from the included stations were re-sectioned into five 5 µm sections for additional study by IHC.
IHC was carried out in the Bond Autostainer (Leica Biosystems Inc., Norwell, MA). Slides were dewaxed in Bond Dewax solution (AR9222) and hydrated in Bond Wash solution (AR9590). Antigen retrieval was performed for 30 min at 100 °C in Bond-Epitope Retrieval solution1 pH-6.0 (AR9961). After pretreatment, the slides were incubated with mouse monoclonal anti-human cytokeratin (AE1/AE3) (Dako, M3515, Agilent Pathology Solutions, Santa Clara, CA 95051) diluted 1:200 for 30 min. Detection was performed using Bond™ Polymer Refine Detection (DS9800). Stained slides were dehydrated and cover-slipped. The same positive and negative (no primary antibody) control tissues were included for each staining batch. All slides were then reviewed for evidence of MMs by a pathologist (JH) blinded to the clinical scenario. LN MMs were defined as one or more non-bronchial epithelial abnormal cells which showed strong membranous positive staining for cytokeratin.
Data abstraction
The date of diagnosis was defined as the date of the first positive specimen obtained, either from EBUS-TBNA, CT-guided biopsy, navigational bronchoscopy, or surgery. Time to progression and time to death are reported with respect to the date of diagnosis. The date of death for a patient was obtained from either the medical record directly or a search of public records. If no record of the patient’s death was available, they were censored on the last date of contact available through the medical record. Staging was determined using the 7th edition of the International Association for the Study of Lung Cancer TNM system (21). Clinical staging was reported with respect to the date of EBUS-TBNA performance (i.e., LN metastases during subsequent surgical resection were not included in the reported stage). Tumor sizes were abstracted from surgical resection specimen reports when available or measurements from the most recently available cross-sectional imaging with respect to the date of diagnosis.
Statistical considerations
Data collection and analysis was performed using GraphPad Prism version 7.04 for Windows (GraphPad Software, La Jolla California USA). Data are reported as the absolute number and percent of the total for categorical variables, the median survival probability and standard error of measurement, or the median and interquartile range (Q1–Q3) for the remaining continuous variables. Survival analysis was performed for overall mortality and progression free survival for all patients and for clinical stage I and II patients only using Kaplan-Meier estimates; the survival functions were compared using a log-rank test.
Results
A total of 887 patients were screened for study inclusion of whom 389 underwent a qualifying EBUS procedure. Of the 389 patients identified, 228 patients were excluded because the EBUS detected N2 or N3 disease or the patient was metastatic at the time of the procedure. An additional 117 patients were excluded because either a non-malignant diagnosis was obtained or no diagnosis of NSCLC was obtained in the required 3-month window surrounding the EBUS-TBNA, or insufficient tissue was available for additional testing. A total 44 patients were identified meeting inclusion criteria with sufficient additional tissue for testing and were further analyzed.
Patient demographics are summarized in Table 1. The study sample contained exclusively Caucasians and African Americans. A majority of patients were male. Three patients were lifelong non-smokers, however, the vast majority of patients had a heavy smoking history. EBUS procedure, clinical staging, and treatment data are summarized in Table 2. The median number of LN stations sampled during EBUS-TBNA was 3. Curative intent therapy was attempted in 41 patients (93%).

Full table

Full table
Three patients (6.8%) had detectible MM in their EBUS-TBNA specimens (Figure 1). The MMs were found in N2 nodes only. The 3 patients found to have MM were clinically staged as IA, IB, and IIA; only the stage IIA patient had a tumor larger than 4 cm. The stage IA and stage IB patients’ primary tumors were in the right upper lobe and the stage IIA patient’s tumor was located in the left lower lobe. The clinically-staged IIA patient underwent surgical resection during which a positive N2 LN was detected (station 9) at the time of resection; the MMs in that patient were detected on the EBUS-TBNA specimens at station 8 and 11L. This patient was included in the survival analysis based on their clinical stage to remain consistent with the information that would be available based on the MM analysis of their EBUS-TBNA samples. The stage IA patient was treated with SBRT and the stage IB patient was treated with chemotherapy and conventional radiation therapy as the lesion was deemed too close to the esophagus for treatment with SBRT.
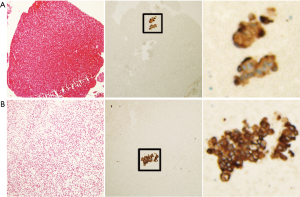
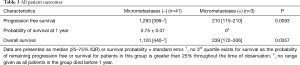
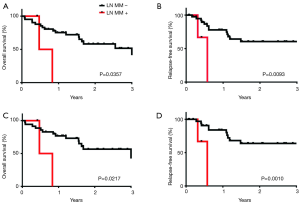
The presence of detectible micrometastases was associated with significantly worsened overall mortality and progression-free mortality in all patients (Table 3) and when limiting the analysis to stage 1 and 2 patients only (Table 4, Figure 2).
Full table

Full table
Discussion
Our findings suggest that NSCLC LN MMs can be detected using IHC staining for pan-cytokeratin in EBUS-TBNA specimens, and that their presence is associated with a worsened progression-free and overall survival. This finding is important as EBUS-TBNA is routinely used as a first line staging modality for invasive mediastinal staging in multiple clinical scenarios, including in patients with a high likelihood of N2 or N3 disease and in patients unable or unwilling to undergo surgical therapy but in whom definitive radiation therapy is planned (12,18,19).
Several groups have examined endoscopic fine needle aspiration specimens of the LNs of NSCLC patients for the presence of MM using ancillary techniques such as reverse transcription polymerase chain reaction (RT-PCR) and flow cytometry (22-25). These studies were conducted with the premise that the addition of RT-PCR or flow cytometry to standard H+E staining of the endoscopic FNA specimens could increase the sensitivity of detection. Wallace et al. examined endoscopic ultrasound-guided aspirations of mediastinal LNs for the presence of MM by measuring expression of a 6-gene panel. They detected relevant expression of at least 1 of the 6 genes in 30% of patients (22). RT-PCR detection of members of the melanoma antigen proteins (MAGE) was reported in the EBUS-TBNA specimens of 8% to 49% of LNs by one group (23,24). The addition of RT-PCR data from MAGE 1–6 and 12 expression to standard H+E staining of EBUS-TBNA samples increased the sensitivity of the procedure by 11.6% (24). Another group reported that examination of hypermethylation of the human homeobox gene SHOX2 by RT-PCR increased the sensitivity and specificity of EBUS-TBNA when combined with standard H+E staining (25). Notably, none of these studies correlated their findings to IHC evaluation of the FNA specimen or patient outcomes. As such, the significance of these findings is unclear, particularly in light of their relatively high rate of detection of potential MMs.
Two recent large scale, prospective trials have evaluated the prevalence and significance of LN MM in surgically resected specimens. Rusch et al. evaluated 1,047 patients with previously untreated, resectable stage I to IIIb disease deemed LN metastasis negative by H+E. LN MM were detected by IHC in 22.4% of patients; the presence of MMs was associated with worsened overall and disease-free survival (10). Martin et al. evaluated 298 patients with suspected stage I NSCLC and found that LN MMs were detectable in 13.8% by IHC. They reported that patients with LN MM at N2 stations had worse survival rates. In addition, 69% of LNs were positive for MM using RT-PCR to evaluate for CEA expression. There was no correlation between CEA expression and patient outcomes (11).
Our study found detectable LN MMs in 6.8% of the patients evaluated who were originally thought to be metastasis-free on initial EBUS-TBNA. There are several possible reasons for the discrepancy between our reported incidence of LN MM detection and that reported in the prospective surgical literature (14–22% incidence). Our sample size is small and the discrepancy may be the result of random variation. It is unclear if the deparaffinization required to process our specimens affects the detection of MMs. Sampling error of the EBUS-TBNA procedure is another possibility. FNA samples sample a much smaller proportion of tissue as compared with the entire excised LN. A recent analysis of 89 LNs sampled via EBUS-TBNA and subsequently surgically resected demonstrates two instances of documented sampling error in LNs biopsied via EBUS-TBNA in which MMs were discovered (26). While it is difficult to extrapolate those findings directly to our data, it does suggest that sampling error may have some role in the discrepancy as well. Given the significant association we found between the presence of LN MMs and poor prognosis, there may be benefit of the additional information garnered in those patients with detected LN MMs. The number of LN stations sampled in our study is consistent with that reported by Martin et al. in which 81% of patients had at least 3 LN stations sampled and submitted for central review. As such, LN sampling frequency is unlikely to explain the discrepancy.
There are several important limitations to consider for this work. First, no matched surgical samples are available from the EBUS-TBNA samples to serve as a gold standard. Second, bronchial epithelial cells can be contaminants of EBUS-TBNA samples as the needle must pass through the bronchial wall to sample LNs. Bronchial epithelial cells stain positively with CK antibodies in cell block samples. However, benign bronchial epithelium is usually easily distinguished from malignancy by an experienced cytopathologist. It is worth noting that CK19 mRNA was detected in 98% of EBUS-TBNA samples evaluated with RT-PCR by one group, consistent with some level of contamination of the specimens (23). The worsened disease-free and overall survival outcomes noted in our LN MM-positive patients argues against contamination as the explanation for our findings and bolsters the argument that pan-cytokeratin positive cells represent true LN MMs. Perhaps further exploration of the use of RT-PCR can help increase the specificity of this technique without compromising future sensitivity. Other limitations include the lack of prospective evaluation and the semi-systematic patient selection methodology. Although the patient’s LN MM status and outcome were not known at the time of selection for inclusion a selection bias risk remains.
In summary, we have demonstrated that LN MMs can be detected in a subset of NSCLC patients sampled with EBUS-TBNA using pan-cytokeratin IHC. In addition, the presence of occult LN MMs is associated with significantly worse prognosis in this small study. Further work should focus on the validation of these findings in a larger prospective cohort with a focus on the non-surgical candidate population and methods for improving the sensitivity of MM detection via EBUS-TBNA.
Acknowledgments
The authors would like to thank the UNC Translational Pathology Laboratory for technical support.
Funding: This work was supported by a grant from the Lung Cancer Initiative of North Carolina [5101317]. The LCI of NC was not involved in any element of study design, execution, or reporting. CV Pecot was supported in part by the National Institutes of Health R01-CA215075, R01-CA042978 and U54-CA198999, a Mentored Research Scholar Grants in Applied and Clinical Research (MRSG-14-222-01-RMC) from the American Cancer Society, the Jimmy V Foundation Scholar award, the UCRF Innovator Award, the Stuart Scott V Foundation/Lung Cancer Initiative Award for Clinical Research, the University Cancer Research Fund, the Lung Cancer Research Foundation, the Free to Breathe Metastasis Research Award and the Susan G. Komen Career Catalyst Award.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the University of North Carolina at Chapel Hill Program Review Committee and Institutional Review Board (LCCC 1425 and 14-1755, respectively).
References
- Institute NC (2018). "SEER Cancer Statistics Factsheets: Lung and Bronchus Cancer." Retrieved 6/5/2018. Available online: http://seer.cancer.gov/statfacts/html/lungb.html
- Taylor MD, Nagji AS, Bhamidipati CM, et al. Tumor recurrence after complete resection for non-small cell lung cancer. Ann Thorac Surg 2012;93:1813-20; discussion 1820-1.
- Lou F, Sima CS, Rusch VW, et al. Differences in patterns of recurrence in early-stage versus locally advanced non-small cell lung cancer. Ann Thorac Surg 2014;98:1755-60; discussion 1760-1.
- Giuliani ME, Hope A, Mangona V, et al. Predictors and Patterns of Regional Recurrence Following Lung SBRT: A Report From the Elekta Lung Research Group. Clin Lung Cancer 2017;18:162-8. [Crossref] [PubMed]
- Wink KCJ, van Baardwijk A, Troost EGC, et al. Nodal recurrence after stereotactic body radiotherapy for early stage non-small cell lung cancer: Incidence and proposed risk factors. Cancer Treat Rev 2017;56:8-15. [Crossref] [PubMed]
- Chen ZL, Perez S, Holmes EC, et al. Frequency and Distribution of Occult Micrometastases in Lymph-Nodes of Patients with Non-Small-Cell Lung-Carcinoma. J Natl Cancer Inst 1993;85:493-8. [Crossref] [PubMed]
- Passlick B, Izbicki JR, Kubuschok B, et al. Immunohistochemical assessment of individual tumor cells in lymph nodes of patients with non-small-cell lung cancer. J Clin Oncol 1994;12:1827-32. [Crossref] [PubMed]
- Izbicki JR, Passlick B, Hosch SB, et al. Mode of spread in the early phase of lymphatic metastasis in non-small-cell lung cancer: significance of nodal micrometastasis. J Thorac Cardiovasc Surg 1996;112:623-30. [Crossref] [PubMed]
- Kubuschok B, Passlick B, Izbicki JR, et al. Disseminated tumor cells in lymph nodes as a determinant for survival in surgically resected non-small-cell lung cancer. J Clin Oncol 1999;17:19-24. [Crossref] [PubMed]
- Rusch VW, Hawes D, Decker PA, et al. Occult metastases in lymph nodes predict survival in resectable non-small-cell lung cancer: report of the ACOSOG Z0040 trial. J Clin Oncol 2011;29:4313-9. [Crossref] [PubMed]
- Martin LW, D'Cunha J, Wang X, et al. Detection of Occult Micrometastases in Patients With Clinical Stage I Non-Small-Cell Lung Cancer: A Prospective Analysis of Mature Results of CALGB 9761 (Alliance). J Clin Oncol 2016;34:1484-91. [Crossref] [PubMed]
- Silvestri GA, Gonzalez AV, Jantz MA, et al. Methods for staging non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e211S-50S.
- Tammemagi CM, Neslund-Dudas C, Simoff M, et al. Impact of comorbidity on lung cancer survival. Int J Cancer 2003;103:792-802. [Crossref] [PubMed]
- Alexander M, Evans SM, Stirling RG, et al. The Influence of Comorbidity and the Simplified Comorbidity Score on Overall Survival in Non-Small Cell Lung Cancer-A Prospective Cohort Study. J Thorac Oncol 2016;11:748-57. [Crossref] [PubMed]
- Wisnivesky JP, Bonomi M, Henschke C, et al. Radiation therapy for the treatment of unresected stage I-II non-small cell lung cancer. Chest 2005;128:1461-7. [Crossref] [PubMed]
- Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA 2010;303:1070-6. [Crossref] [PubMed]
- Lischalk JW, Woo SM, Kataria S, et al. Long-term outcomes of stereotactic body radiation therapy (SBRT) with fiducial tracking for inoperable stage I non-small cell lung cancer (NSCLC). J Radiat Oncol 2016;5:379-87. [Crossref] [PubMed]
- Steinfort DP, Siva S, Leong TL, et al. Systematic Endobronchial Ultrasound-guided Mediastinal Staging Versus Positron Emission Tomography for Comprehensive Mediastinal Staging in NSCLC Before Radical Radiotherapy of Non-small Cell Lung Cancer: A Pilot Study. Medicine (Baltimore) 2016;95:e2488. [Crossref] [PubMed]
- Vial MR, Khan KA, O'Connell O, et al. Endobronchial Ultrasound-Guided Transbronchial Needle Aspiration in the Nodal Staging of Stereotactic Ablative Body Radiotherapy Patients. Ann Thorac Surg 2017;103:1600-5. [Crossref] [PubMed]
- Gilbert C, Yarmus L, Feller-Kopman D. Use of endobronchial ultrasound and endoscopic ultrasound to stage the mediastinum in early-stage lung cancer. J Natl Compr Canc Netw 2012;10:1277-82. [Crossref] [PubMed]
- Goldstraw P, Crowley J, Chansky K, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM Classification of malignant tumours. J Thorac Oncol 2007;2:706-14. [Crossref] [PubMed]
- Wallace MB, Block MI, Gillanders W, et al. Accurate molecular detection of non-small cell lung cancer metastases in mediastinal lymph nodes sampled by endoscopic ultrasound-guided needle aspiration. Chest 2005;127:430-7. [Crossref] [PubMed]
- Dango S, Cucuruz B, Mayer O, et al. Detection of disseminated tumour cells in mediastinoscopic lymph node biopsies and endobronchial ultrasonography-guided transbronchial needle aspiration in patients with suspected lung cancer. Lung Cancer 2010;68:383-8. [Crossref] [PubMed]
- Cucuruz B, Dango S, Jurinovic V, et al. MAGE qPCR improves the sensitivity and accuracy of EBUS-TBNA for the detection of lymphatic cancer spread. J Thorac Oncol 2012;7:690-7. [Crossref] [PubMed]
- Darwiche K, Zarogoulidis P, Baehner K, et al. Assessment of SHOX2 methylation in EBUS-TBNA specimen improves accuracy in lung cancer staging. Ann Oncol 2013;24:2866-70. [Crossref] [PubMed]
- Khazai L, Kundu UR, Jacob B, et al. Endobronchial ultrasound-guided transbronchial needle aspiration biopsy is useful evaluating mediastinal lymphadenopathy in a cancer center. Cytojournal 2011;8:10. [Crossref] [PubMed]


