Adjuvant therapy for pathological T3N0M0 esophageal squamous cell carcinoma
Introduction
Primary resection is not recommended by National Comprehensive Cancer Network (NCCN) guideline for squamous cell esophageal cancer (ESCC) invading the adventitia (T3) and neoadjuvant chemoradiotherapy followed by surgery is the standard remedy for these patients (1). The series of results of CROSS trial had showed that patients with esophageal cancer would benefit from neoadjuvant chemoradiotherapy followed by esophagectomy (2). However, in China, despite of the increased rate of neoadjuvant therapy, patients would prefer to primary esophagectomy than neoadjuvant therapy followed with esophagectomy, especially when the clinical evaluation of lymph nodes metastasis is negative. The reasons may due to the surgeon’s preference of trimodality therapy and patients’ will. Another reason may due to the underestimate of clinical stage for locally advanced (T3-4) esophageal cancer (3). Nevertheless, the advantage of neoadjuvant versus adjuvant has not been widely reported (4).
Adjuvant therapy is not indicated for patients without node metastasis (pN0) after primary surgery (1,5). But the consideration of adjuvant treatment for pathological T3N0 (pT3N0) stage is controversial (6). The pT3 is a strong risk factors for recurrence, even when the N stage is negative (5). The purpose of this study was to determine the role of postoperative therapy for the recurrence prevention and survival improvement in patients with pT3N0M0 ESCC.
Methods
Patients selection
The retrospective research enrolled patients undergoing esophagectomy for esophageal cancer at Shanghai Chest Hospital from January 2012 to September 2014. Selection criteria were defined as: (I) patients with pT3N0M0 ESCC according to the 7th edition of the Union for International Cancer Control esophageal cancer staging system; (II) Eastern Cooperative Oncology Group (ECOG) performance status was 0–2. The exclusion criteria were: (I) non-R0 resection; (II) history of neoadjuvant chemoradiotherapy or molecular targeted therapy; (III) death related to the surgery within 3 months; (IV) with other pathological malignancy diagnoses or with other active secondary malignancy.
A total of 200 patients were enrolled, and were divided into two groups, including 111 cases in surgery alone group (Group S) and 89 cases in surgery plus adjuvant chemo/radiation/chemoradiation therapy group (Group S + aCRT).
Surgery
All patients underwent McKeown esophagectomy with at least a two-field (thoracic and abdominal) lymphadenectomy. The category of regional lymph nodes followed Japanese classification (7). The dissected nodes station should include bilateral recurrent laryngeal nerve, upper esophagus, para-hilum, middle and lower esophagus, upper diagram, para-esophago-gastric area, celiac area and lesser curve. The Stomach was used for all patients as conduit reconstruction.
Surgical pathological evaluation
The specimen was evaluated by two pathologists in our institution. The pathological T3 was defined with tumor invading to the esophageal adventitia, but the circumferential margin was negative.
Adjuvant therapy
In our institution, for patients with ESCC who accepted initial esophagectomy, the principle of administration of adjuvant chemoradiation (aCRT) is that: pathological T1a-3N1-3 and T4a-4bNx, R1, lymphavascular invasion and tumor located at the neck. For patients with pT3N0M0, the indications of adjuvant therapy depended on the attending surgeons' inclination and patients’ selection.
Of 89 patients in Group S + aCRT, adjuvant therapy started 4–6 weeks after surgery. The full courses were defined with at least two cycles of chemotherapy and a sequential dose of 50–50.4 Gy radiation.
Docetaxel at a dosage of 60 mg/m2 of a body-surface area and cisplatin was administered at a dose of 70 mg/m2 was given on day 1. A total of 2–4 courses of chemotherapy were given, each course of chemotherapy lasted 28 days (4 weeks).
A sequential radiotherapy was given 2–4 week after the completion of chemotherapy. Radiotherapy was delivered with photons (6 MV) to a total dose of 50–50.4 Gy in 25–28 fractions. Patients were treated 5 days per week at 1.8–2 Gy/d. Intensity modulated radiotherapy based on CT simulation planning system with 5-mm-thick scan slice throughout the entire neck and thorax was required. The clinical target volume was designed as a T-shaped field that encompassed the bilateral supraclavicular fossa, superior mediastinum and subcarinal area wherever the primary tumor located.
Parameters and follow-up
Overall survival (OS) was measured from the date of surgery to the date of death or last follow-up and censored at the last contact date in surviving patients. Disease-free survival (DFS) was measured from the date of surgery to the date of first evidence of relapse or death from any cause, which was observed first. For patients who had not relapsed or died, DFS was censored at the last follow-up date. The last date of follow-up was at August 30, 2018 in this study. Recurrence was confirmed and recorded by means of physical examination, CT scan of chest and abdomen, ultrasound of the neck, and PET-CT scan.
Statistical analysis
The differences in clinical and pathological characteristics between Group S and Group S + aCRT were assessed by the chi-square test. OS and DFS curves were presented by Kaplan-Meier method with log-rank test for comparisons of two treatment groups. A two-tailed P<0.05 was considered statistically significant. Analysis was performed with the SPSS statistical software package version 22.0.
Results
Patients’ demographics
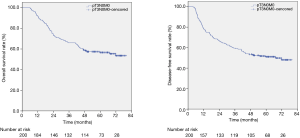
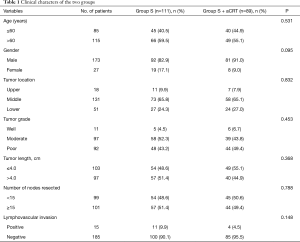
During the period from January 2012 to September 2014, a total of 200 consecutive patients with surgically pathological stage of T3N0M0 were enrolled in this study with a mean age of 61.6±7.8 years, including 173 males and 27 females. These patients comprised 25.4% of all patients (200 of 787) with resection of esophageal cancer in Shanghai Chest Hospital. Eighty-nine (44.5%) patients received adjuvant chemo/radiotherapy (Group S + aCRT), while the other 111 (55.5%) patients underwent surgery alone (Group S). In Group S + aCRT, the full course adjuvant treatment was used in 23.6% of patients, and the rest, 33.7% of patients accepted chemotherapy alone and 42.7% underwent radiation alone. No remarkable difference was observed in the age, gender, tumor location, tumor length, number of lymph nodes resected and incidence of lymphavascular invasion between two groups. Table 1 showed patients’ demographics. No difference was found in postoperative complications and in-hospital days between two groups.
Full table
DFS and OS
Among all patients, the median follow-up was 63.3 months (range, 47.1 to 79.7 months). Eighty-eight deaths were observed during the follow-up and 74 patients experienced recurrence. Analyzing the whole cohort, DFS curves are shown in Figure 1. The 3 and 5-year DFS rates were 59.5% and 51.1%, and the 3 and 5-year OS rates were 66.0% and 56.6%, respectively.
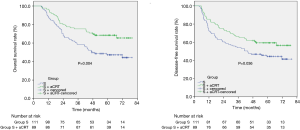
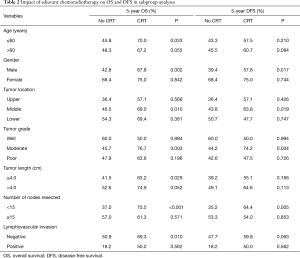
The 5-year OS rate was 47.2% in Group S and 68.4% in Group S + aCRT (P=0.004, Figure 2A). The results of subgroup analysis on OS showed in Table 2, the results of subgroup analysis on OS showed that the 5-year OS was improved by adjuvant therapy in the subgroups of age ≤60 (P=0.033), males (P=0.002), tumor located at the middle of thorax (P=0.010), moderate differentiation (P=0.003), tumor length ≤4.0 cm (P=0.029), number of resected lymph nodes <15 (P<0.001) and negative lymphavascular invasion (P=0.010).
Full table
The 5-year DFS rate was 44.4% in Group S and 59.3% in Group S + aCRT (P=0.036, Figure 2B). DFS outcomes stratified by subgroup are summarized in Table 2. The results of subgroup analysis on DFS showed that the 5-year DFS was improved by adjuvant therapy in the subgroups of males (P=0.017), tumor located at the middle of thorax (P=0.019), moderate differentiation (P=0.004) and number of resected lymph nodes <15 (P=0.005).
Univariate and multivariate analysis for OS and DFS
In order to find out the independent prognostic factor of OS and DFS for patients with pT3N0M0, we did univariate and multivariate analysis. On univariate analysis of OS, lymphavascular invasion and adjuvant chemoradiotherapy (P=0.010, 0.004 respectively) were related to the OS, and further multivariate analysis was performed with including variables of gender, tumor location, lymphavascular invasion and adjuvant chemoradiotherapy. The results showed that positive lymphavascular invasion (HR 2.06, 95% CI: 1.11–3.81, P=0.021) was associated with increased risk of death, while female (HR 0.47, 95% CI: 0.23–0.98, P=0.044) and adjuvant chemoradiotherapy (HR 0.53, 95% CI: 0.34–0.83, P=0.005) was associated with improved OS (Table 3).
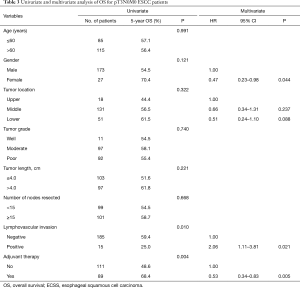
Full table
On univariate analysis of DFS, gender, lymphavascular invasion and adjuvant chemoradiotherapy (P=0.045, 0.030, 0.036, respectively) were related to DFS. The results of multivariate analysis showed that female (HR 0.45, 95% CI: 0.22–0.94, P=0.032), as well as adjuvant chemoradiotherapy (HR 0.63, 95% CI: 0.42–0.95, P=0.028) were associated with improved DFS (Table 4).
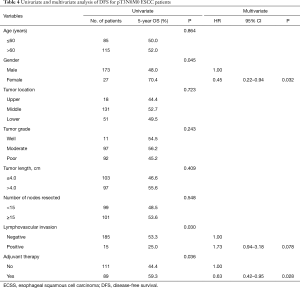
Full table
Recurrence pattern
Cancer recurrence developed in 29 (32.6%) patients in Group S + aCRT and in 45 (40.5%) patients in Group S. Table 5 showed the recurrent location. The frequency of locoregional recurrences, particularly in the cervical and mediastinal nodes, was higher in Group S than in Group S + aCRT (27.0%, 30 in Group S vs. 13.5%, 12 in Group S + aCRT, respectively, P=0.019). And 21 (72.4%) underwent local or systemic treatments for recurrence in Group S + aCRT and 27 (60.0%) of 45 patients in Group S. There was no statistical difference in the subgroup comparison analysis of adjuvant radiation (Group S + RT) and adjuvant chemotherapy (Group S + CT) and adjuvant chemoradiation (Group S + CRT) in terms of locoregional recurrence (P>0.05) or distant metastasis (P>0.05).
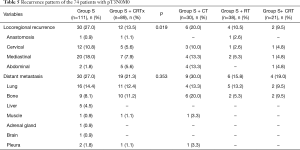
Full table
Discussion
Adventitia invasion is the most common T stage for clinical diagnosis of esophageal cancer. Current report was a large retrospective cohort study for the impact of adjuvant chemoradiotherapy on the OS and DFS in patients with pT3N0M0 ESCC. Our results showed that adjuvant chemo/radiotherapy could improve the 5-year DFS and OS for these patients. This benefit may be due to clinical underestimation of patients with potential progression, as well as timely rescue through adjuvant therapy.
The lymph node metastasis (LNM) rate is highly related to the invasion depth of tumor in esophageal cancer. The trend of increasing LNM rate with increasing depth of invasion is obvious (8). This law also appears in our previous study (9). In pathological T3 (pT3) cases, even the nodes are negative (pN0), we still can observe a very high incidence of late recurrence (43.4%) (10). This relapse rate is higher than the pT1-T2N0 case. Therefore, we have every reason to believe that patients with stage T3, whether with or without lymph node metastasis, are at high risk, and multimodality therapy is necessary.
Neoadjuvant chemoradiation (nCRT) followed with esophagectomy is now widely accepted as the standard treatment for locally advanced esophageal cancer due to the benefit of tumor downstage, promoted radical resection, and in favor of improved survival (4,11,12). The CROSS trial showed that after nCRT, 23% of patients with adenocarcinoma (AD) and 49% of patients with squamous cell carcinoma (ESCC) have a pathologically complete response (pCR) in the resection specimen and improved R0 resection compared to surgery alone (2). The long-term results had demonstrated that compared to surgery alone, a significant prolonged OS time and lower recurrent rate were found in the nCRT plus surgery group, particularly for patients with squamous cell carcinoma (11).
Postoperative adjuvant therapy is also believed to have benefit on local tumor control and promoted long-term survival for patients who accepted initial esophagectomy. The JCOG9204 study from Japan, aimed to determine whether postoperative adjuvant chemotherapy improves outcome in patients with ESCC undergoing initial radical esophagectomy. And the results showed that patients would benefit from postoperative chemotherapy regarding to the 5-year recurrence free survival time, especially for patients with pathologically positive lymph nodes (4). In a prospective randomized study, 549 patients with ESCC were randomized into surgery alone group and surgery plus adjuvant radiotherapy group. Postoperative radiotherapy was found to improve the survival of patients with positive lymph nodes and reduce the incidence of intrathoracic recurrence and supraclavicular lymph node metastasis (13).
The JCOG 9907 study compared the preoperative chemotherapy and postoperative chemotherapy in patients with clinical stage II or III ESCC. Preoperative chemotherapy was demonstrated to have superiority of 5-year OS rate (55% vs. 43%, P=0.04) and comparable postoperative complications (14). However, a conflict result was conducted in another prospective study. A total of 238 patients with stage II or III ESCC were randomized into 3 groups: preoperative CRT (80 cases), postoperative CRT (78 cases) and surgery alone (S) (80 cases). Pre-CRT and Post-CRT would provide benefit of OS and progressive-free survival (PFS) than surgery alone, but no long-term survival difference was found between Pre-CRT and Post-CRT (4).
The role of chemoradiation in the multi-treatment of esophageal cancer is certain no matter of in the preoperative stage or postoperative stage. How to select the proper patients with ESCC that could benefit from optimal multi-treatment strategy is still in research. In this study, we attempted to find the role of adjuvant therapy in patients with pT3N0M0. The advantage of postoperative adjuvant therapy is that the administration is based on the accurate pathological staging.
In clinical practice, one reason for the preferred primary esophagectomy by surgeons is that the worry of tumor progression during preoperative chemoradiation, resulting in losing radical surgery opportunity. However, for patients who accepted nCRT and developed to distant metastasis prior to esophagectomy, as well as patients who accepted initial esophagectomy and experienced early distant metastasis, they would have tumor progression with their inherent tumor biology without any potential benefit from esophagectomy instead of high risk of morbidity and mortality. In this way, preoperative chemoradiation is primary recommended as first line treatment, especially for patients with locally advanced esophageal cancer. Several studies have described post-neoadjuvant therapy metastases with an incidence of 8–17% (15-17). And in our study, 5 (2.5%) patients experienced early distant metastasis within 3 months after esophagectomy.
However, the full implementation of this strategy is limited by socio-economic conditions in China, although it has been significantly improved now. Adjuvant therapy is a compromised selection for them. In JCOG9204 study from Japan, adjuvant therapy was proved valuable only for the patients with positive lymph nodes (3). But the sample size of T3N0 was small in their study. In the present study, more than 200 such cases were reviewed.
The prognostic effect of the number of resected lymph nodes on patients with esophageal cancer has been still ongoing investigation. Two retrospective studies had demonstrated that the number of resected lymph nodes is an independent prognostic factor for survival (18,19), and one retrospective study identified that at least 15 resected lymph nodes are required for adequate staging for patients with esophageal cancer undergoing surgery with or without preoperative CRT (20). In our subgroup analysis, the patients with number of resected lymph nodes <15 (P<0.001) can be improved in OS by adjuvant therapy. Few lymph node dissections can prompt two questions: first, radical resection is not enough, the risk of postoperative recurrence is increased; second, the staging of the tumor is underestimated. There is no clear definition of how many quantities of nodes are enough for radical lymphadenectomy, which can be affected by many factors, such as surgical techniques and pathology testing capabilities (21-24). In the NCCN guideline, in patients undergoing esophagectomy without induction chemoradiation, at least 15 lymph nodes should be removed to acquire adequate nodal staging. Therefore, for the patients with low number of nodes, adjuvant therapy is mandatory to make up for the lack of surgery.
The other subgroup characteristics to approve the value of adjuvant therapy for survival were as follow: young age (<60 years old), male gender, middle thoracic location, moderate differentiation, short tumor length (≤4.0 cm), and negative lymphavascular invasion. The exact benefit of the subgroup of people with these characteristics is still unknown. However, we speculate that better tolerance for postoperative adjuvant therapy is the main reason for their benefit.
How to select a suitable adjuvant therapy was difficult. Wong and colleagues (25) analyzed 4,893 patients with stage pT3-4Nx-0M0 (localized) or pT1-4N1-3M0 (regional) esophageal cancer (squamous cell carcinoma or adenocarcinoma) from the National Cancer Database. The results showed that for patients with pT3-4Nx-0M0 and negative margin, the 3-year OS was not found difference between surgery alone and surgery plus postoperative radiation (41.3% vs. 46.9%, P=0.24), while for patients with positive margin, postoperative radiation increased the survival status. In our study, we did not do subgroup analysis for chemotherapy, radiation, and chemo-radio therapy. In addition to lymph node metastasis, many factors influence the choice of postoperative adjuvant therapy, such as lymphavascular invasion, tumor location, differentiation, and subjective assessment of surgical procedures by surgeons. In our clinical practice, tumor location is the most important factor affecting our choice of radiation therapy for patients with pN0. If the tumor locates in the upper or middle thoracic esophagus, an adjuvant radiotherapy encompassing the neck (the bilateral subclavicular regions), superior mediastinum, and sub-carina is routinely recommended. It will also be combined with chemotherapy. Because the upper and middle esophageal cancers have higher possibility of upper mediastinal and cervical lymph node metastasis, and these two areas are also the most difficult areas for lymph node dissection by surgery (26-29). However, for the lower esophageal cancer, lymph node metastasis is concentrated in esophagogastric junction, celiac trunk, and lesser curve of stomach. The complete surgical resection rate is very high at above areas, so regional adjuvant radiotherapy is not very important. Even with the same adjuvant radiotherapy, the target area will still choose the small “T” field in the upper mediastinum and neck
This is only a retrospective study. The major limitation of this study was that administration of postoperative adjuvant therapy was affected by some bias, including 8 surgeons’ different preference of trimodality treatment in this cohort, patients’ worry of side effects of adjuvant therapy and economical status and the impact of these and other possible sources of bias could not be precisely evaluated. Another limitation is that we didn’t perform exact subgroup analysis of radiotherapy, chemoradiotherapy and chemotherapy, and there are many interference factors. There are no good alternatives to make good subgroup analysis due to the heterogeneity of the data. However, this is a large group of studies on postoperative adjuvant therapy in patients with pT3N0M0. The preoperative characteristics has good consistency between two groups and can respond to the effects of adjuvant therapy. A prospective randomized controlled study is needed in the future.
Conclusions
Postoperative adjuvant therapy could improve DFS and OS in patients with pT3N0M0 ESCC, especially in patients with insufficient lymph node dissection (<15).
Acknowledgments
Funding: This work was supported by Three Years of Clinical Innovation Action Plan (16CR1035B).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the Ethics Committee of Shanghai Chest Hospital (No. KS1903) and written informed consent was obtained from all patients.
References
- Rice TW, Ishwaran H, Ferguson MK, et al. Cancer of the Esophagus and Esophagogastric Junction: An Eighth Edition Staging Primer. J Thorac Oncol 2017;12:36-2.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Esophageal Cancer Study Group Participating Centers. Predictors of staging accuracy, pathologic nodal involvement, and overall survival for cT2N0 carcinoma of the esophagus. J Thorac Cardiovasc Surg 2018. [Epub ahead of print]. [PubMed]
- Lv J, Cao XF, Zhu B, et al. Long-term efficacy of perioperative chemoradiotherapy on esophageal squamous cell carcinoma. World J Gastroenterol 2010;16:1649-54. [Crossref] [PubMed]
- Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol 2003;21:4592-6. [Crossref] [PubMed]
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part I. Esophagus 2017;14:1-36.
- Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus 2011;24:33-8. [Crossref] [PubMed]
- Ye B, Zhong CX, Yang Y, et al. Lymph node dissection in esophageal carcinoma: Minimally invasive esophagectomy versus open surgery. World J Gastroenterol 2016;22:4750-6. [Crossref] [PubMed]
- Wang Y, Wang L, Yang Q, et al. Factors on prognosis in patients of stage pT3N0M0 thoracic esophageal squamous cell carcinoma after two-field esophagectomy. J Cancer Res Ther 2015;11 Suppl 1:C16-23. [Crossref] [PubMed]
- Shapiro J, van Lanschot JJ, Hulshof MC, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. Lancet Oncol 2015;16:1090-8. [Crossref] [PubMed]
- Huang Y, Wang H, Luo G, et al. A systematic review and network meta-analysis of neoadjuvant therapy combined with surgery for patients with resectable esophageal squamous cell carcinoma. Int J Surg 2017;38:41-7. [Crossref] [PubMed]
- Xiao ZF, Yang ZY, Miao YJ, et al. Influence of number of metastatic lymph nodes on survival of curative resected thoracic esophageal cancer patients and value of radiotherapy: report of 549 cases. Int J Radiat Oncol Biol Phys 2005;62:82-90. [Crossref] [PubMed]
- Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol 2012;19:68-74. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS, Ohja B, et al. The accuracy of endoscopic ultrasonography with fine-needle aspiration, integrated positron emission tomography with computed tomography, and computed tomography in restaging patients with esophageal cancer after neoadjuvant chemoradiotherapy. J Thorac Cardiovasc Surg 2005;129:1232-41. [Crossref] [PubMed]
- Flamen P, Van CE, Lerut A, et al. Positron emission tomography for assessment of the response to induction radiochemotherapy in locally advanced oesophageal cancer. Ann Oncol 2002;13:361-8. [Crossref] [PubMed]
- Weber WA, Ott K, Becker K, et al. Prediction of response to preoperative chemotherapy in adenocarcinomas of the esophagogastric junction by metabolic imaging. J Clin Oncol 2001;19:3058-65. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: An international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Rizk NP, Ishwaran H, Rice TW, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg 2010;251:46-50. [Crossref] [PubMed]
- Mariette C, Piessen G, Briez N, et al. The number of metastatic lymph nodes and the ratio between metastatic and examined lymph nodes are independent prognostic factors in esophageal cancer regardless of neoadjuvant chemoradiation or lymphadenectomy extent. Ann Surg 2008;247:365-71. [Crossref] [PubMed]
- Groth SS, Virnig BA, Whitson BA, et al. Determination of the minimum number of lymph nodes to examine to maximize survival in patients with esophageal carcinoma: data from the Surveillance Epidemiology and End Results database. J Thorac Cardiovasc Surg 2010;139:612-20. [Crossref] [PubMed]
- Altorki NK, Zhou XK, Stiles B, et al. Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 2008;248:221-6. [Crossref] [PubMed]
- Peyre CG, Hagen JA, DeMeester SR, et al. The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 2008;248:549-56. [PubMed]
- Hsu PK, Huang CS, Wang BY, et al. The prognostic value of the number of negative lymph nodes in esophageal cancer patients after transthoracic resection. Ann Thorac Surg 2013;96:995-1001. [Crossref] [PubMed]
- Wong AT, Shao M, Rineer J, et al. The Impact of Adjuvant Postoperative Radiation Therapy and Chemotherapy on Survival After Esophagectomy for Esophageal Carcinoma. Ann Surg 2017;265:1146-51. [Crossref] [PubMed]
- Tachimori Y. Pattern of lymph node metastases of squamous cell esophageal cancer based on the anatomical lymphatic drainage system: efficacy of lymph node dissection according to tumor location. J Thorac Dis 2017;9:S724-30. [Crossref] [PubMed]
- Tachimori Y, Ozawa S, Numasaki H, et al. Efficacy of lymph node dissection by node zones according to tumor location for esophageal squamous cell carcinoma. Esophagus 2016;13:1-7. [Crossref] [PubMed]
- Tanaka H, Ohira M, Kubo N, et al. Association of location of lymph node metastases with postoperative recurrence of esophageal squamous cell carcinoma. Anticancer Res 2012;32:3421-6. [PubMed]
- Liu J, Hu Y, Xie X, et al. Subcarinal node metastasis in thoracic esophageal squamous cell carcinoma. Ann Thorac Surg 2012;93:423-7. [Crossref] [PubMed]