Prognosis after wedge resection in patients with 8th edition TNM stage IA1 and IA2 non-small cell lung cancer
Introduction
The standard treatment of stage I non-small cell lung cancer (NSCLC) has been anatomical lobectomy with mediastinal lymph node dissection (1). However, for smaller (≤2 cm) tumors, the prognosis after sublobar resection (segmentectomy or wedge resection) may be comparable to that of lobectomy, although the results are not conclusive (2,3). At the time of this writing, two ongoing randomized trials (CALGB 140503 and JCOG 0802) were in progress to investigate the hypothesis that sublobar resection is comparable to lobectomy for small-sized (≤2 cm) NSCLC (4,5).
Among sublobar resections, wedge resection is a less complex procedure than segmentectomy, with potential advantages including shorter operation time, less intraoperative blood loss, and better postoperative course. Segmentectomy, on the other hand, requires more sophisticated technique, and is not an easier procedure than lobectomy (6). As a result, the surgical burden after wedge resection should be much smaller. However, it is unclear when wedge resection is applicable for the treatment of small-sized (≤2 cm) lung cancer.
Most recent evaluations of the efficacy of sublobar resection have been based on the 7th edition of the TNM classification and have included tumors smaller than 2 cm. However, the criteria for tumor (T) stage have been updated in the 8th edition of the TNM staging system (7-9). In the 7th edition, the T stage was determined by the maximum size of the entire tumor, while in the 8th edition, the T stage is determined according to the maximum size of the invasive component, without the lepidic component, and the T category has been subdivided according to the size of the invasive component into stages T1a (≤1 cm), T1b (>1 to 2 cm), and T1c (>2 to 3 cm) (8). We wanted to know whether wedge resection is acceptable in a specific stage according to 8th edition of the TNM staging system.
The purpose of this study was to compare the prognoses after wedge resection or lobectomy in patients with stage IA1 and IA2 NSCLC according to the 8th edition of the TNM staging system and to investigate the indications for wedge resection using the 8th TNM staging system, which measures only the invasive component in tumor size.
Methods
Patients
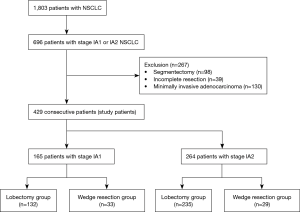
From 2007 to 2017, 1,803 consecutive patients at a tertiary Hospital in Korea were diagnosed with non-small cell lung cancer (NSCLC) and underwent therapeutic surgical resection. Among these patients, 696 were diagnosed with stage IA1 or IA2 NSCLC. Patients with incomplete resection or who had preoperative chemo- or radiotherapy were excluded. We also excluded patients who underwent segmentectomy and those with minimally invasive adenocarcinomas [T1a(mi)N0M0], because it is already known that the prognosis after sublobar resection at this stage is superior to that of other stage I lung cancers. Finally, the study retrospectively enrolled 429 consecutive patients who, according to the 8th edition TNM classification, had TNM stage IA1 and IA2 NSCLC (Figure 1). We compared 5-year disease-free survival (DFS) rates between lobectomy and wedge resection in stage IA1 and IA2 NSCLC and analyzed the risk factors for recurrence after curative resection. We also analyzed the risk factors for recurrence after wedge resection for stage IA1 and IA2 NSCLC in order to identify those cases for which wedge resection would be suboptimal. This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, the Catholic University of Korea (approval ID KC19RESI0064).
Surgical procedures
The first line of treatment for stage I NSCLC is anatomical lobectomy and mediastinal lymph node dissection. However, in patients with ground glass opacity (GGO) or small, solid peripheral nodules near the visceral pleura wedge resection is also considered. The surgical procedure was selected depending on the surgeon’s preference or the patient’s decision, and in the case of high-risk patients with decreased pulmonary function or comorbid disease, wedge resection was usually performed.
Anatomical lobectomy always included mediastinal lymph node dissection at more than 3 mediastinal lymph node stations. Lobectomy on the right side included dissection of paratracheal and subcarinal lymph nodes and lobectomy on the left side included dissection of subaortic and subcarinal lymph nodes. The technique used for lymph node dissection was en bloc resection of the lymph nodes, including adjacent fat tissue.
Wedge resection was performed using endostaplers. Most cases obtained a sufficient resection margin, in which the margin length was greater than the tumor diameter.
Histological evaluation and re-staging to the 8th edition staging system
Pathology reports were reviewed for tumor size, location, lymph node status, and lymphovascular invasion. Lymphovascular invasion was defined as tumor cells microscopically observed in the lymphatic or vascular lumen. Tumor specimens were remeasured by the pathologist to reclassify the T stage according to the 8th edition TNM classification (10), and the T stage was defined by the greatest dimension of the invasive component of the tumor (7). Finally, in the case of wedge resection, the gross cut-surface tumor margin width was measured, and the free resection margin distance was defined as the shortest distance between the tumor and the resection line.
Statistical analysis
Patients were grouped according to tumor stage and surgical procedure. Clinicopathological factors were compared with Student’s t-test or the Wilcoxon rank-sum test for continuous variables and chi-squared or Fisher’s exact test for categorical variables. The interval from surgery to the final follow-up visit was analyzed via the Kaplan-Meier method using confirmed recurrences to calculate 5-year DFS, and survival rates were compared by log-rank test. A Cox proportional hazards model was used in a multivariate analysis to identify risk factors for recurrence of stage IA1 and IA2 NSCLC. All variables with P<0.1 on univariate analysis were entered into the multivariate analysis. A P value of <0.05 was considered statistically significant. Statistical analysis was performed using SPSS version 24.0 software (IBM Corp, Armonk, NY, USA).
Results
Lobectomy versus wedge resection in stage IA1
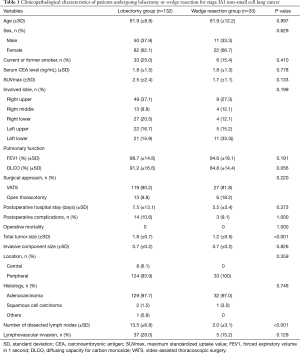
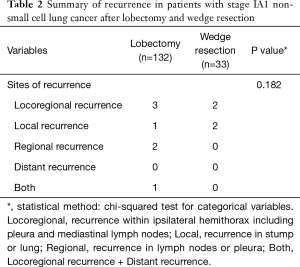
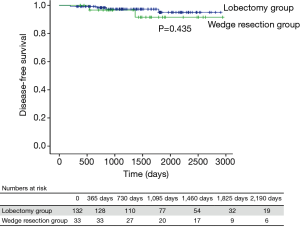
Among patients with stage IA1 NSCLC, 132 underwent lobectomy and 33 underwent wedge resection. There were no significant differences in clinicopathological factors between these 2 groups (Table 1). The median follow-up time for patients with stage IA1 NSCLC was 1,358 days (range, 200–2,942 days), and 6 patients had recurrence (Table 2). The 5-year DFS rate after lobectomy was 95.0%, and 5-year DFS after wedge resection was 91.6% (P=0.435) (Figure 2).
Full table
Full table
Lobectomy versus wedge resection in stage IA2
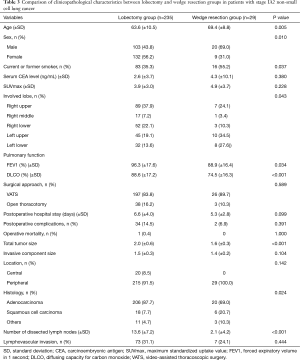
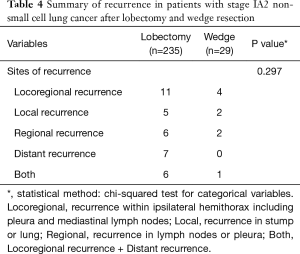
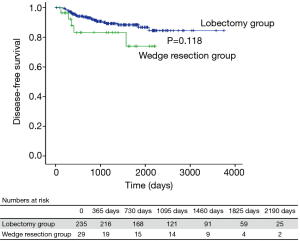
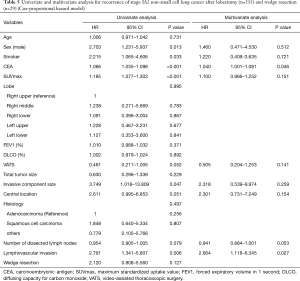
Among patients with stage IA2 NSCLC, 235 underwent lobectomy and 29 had wedge resection. There were significant clinicopathological differences between the lobectomy group and the wedge resection group (Table 3). Older age, male sex, and smoking were more common in the wedge resection group, and pulmonary function was poorer in wedge resection group. Total tumor size, including the lepidic component, was also different between the lobectomy group and the wedge resection group, but the size of the invasive component was not different (1.5 vs. 1.4 cm, P=0.104). Squamous cell carcinoma and other histologic types were more prevalent in the wedge resection group, but the incidence of lymphovascular invasion was not different between the groups. The median follow-up time for patients with stage IA2 NSCLC was 1,224 days (range, 20–3,751 days), and 29 patients had recurrence (Table 4). The 5-year DFS rate was 88.3% after lobectomy and 74.0% after wedge resection (Figure 3). This difference was not statistically significant (P=0.118), but because there were significant between-group differences in clinicopathological characteristics, it was difficult to conclude that lobectomy and wedge resection offered the same prognosis in stage IA2 NSCLC. Therefore, univariate and multivariate analyses using a Cox proportional hazard model were conducted (Table 5). Wedge resection was not a significant risk factor for recurrence on the univariate analysis. Specific variables identified as significant (P<0.1) by univariate analysis included sex, smoking status, serum carcinoembryonic antigen (CEA) level, maximum standardized uptake value (SUVmax), video-assisted thoracoscopic surgery (VATS), invasive component size, central location, number of dissected lymph nodes, and lymphovascular invasion. When these variables were entered into the multivariate model, only the CEA level [hazard ratio (HR) =1.040, P=0.046] and lymphovascular invasion (HR =2.664, P=0.027) were significant risk factors for recurrence of stage IA2 NSCLC.
Full table
Full table
Full table
Risk factors for recurrence after wedge resection
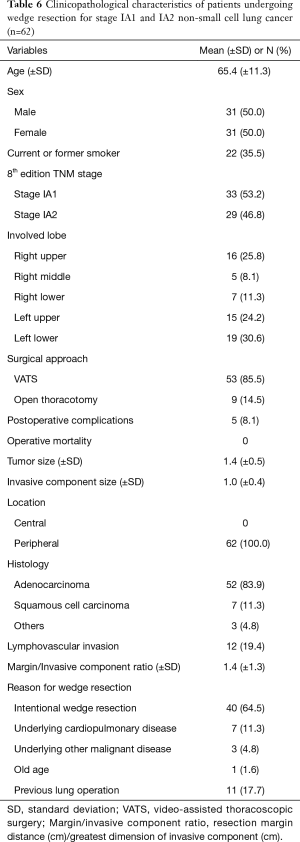
We next analyzed risk factors for recurrence in all patients who had wedge resection (n=62: 33 stage IA1 NSCLC and 29 stage IA2 NSCLC). The clinicopathological characteristics of these patients are shown in Table 6. The mean total tumor size was 1.4 cm and the size of the invasive component was 1.0 cm. The mean margin/invasive component ratio, defined as the resection margin distance (cm)/the greatest dimension of invasive component (cm), was 1.4. Forty patients (64.5%) underwent wedge resection intentionally [25 stage IA1 (75.8%) and 15 stage IA2 (51.7%); P=0.048], while the remaining patients underwent wedge resection because of underlying disease or old age.
Full table
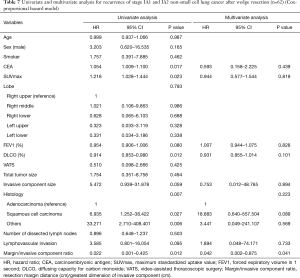
The median follow-up time after wedge resection was 1,294 days (range, 146–2,969 days) and 7 patients had recurrence, 6 with locoregional recurrence and 1 with bone recurrence. Univariate and multivariate analyses using a Cox proportional hazard model were conducted to identify the risk factors for recurrence (Table 7) after wedge resection. Significant factors (P<0.1) on univariate analysis included CEA level, SUVmax, forced expiratory volume in 1 second (FEV1), diffusing capacity for carbon monoxide (DLCO), size of the invasive component, histologic type, lymphovascular invasion, and margin/invasive component ratio. When these variables were entered into the multivariate model, only the margin/invasive component ratio (HR =0.042, P=0.041) was a significant risk factor for recurrence. The mean margin/invasive component ratio in the recurrence group was 0.43 (range, 0.06–1.00), and there were no recurrences when the margin/invasive component ratio was >1.
Full table
Discussion
Wedge resection is a relatively simple and easy procedure in thoracic surgery that is usually associated with a better postoperative course than segmentectomy and lobectomy, along with well-preserved postoperative pulmonary function. Wedge resection is usually indicated for metastasectomy or lung biopsy, and the prognosis after wedge resection has also been good in some cases of lung cancer presenting as GGO nodules (11). Thus, patients with non-invasive or minimally invasive tumors can be candidates for wedge resection. However, it has not been established whether wedge resection is acceptable procedure in NSCLC. Therefore, we evaluated the prognosis of 8th edition TNM stage IA1 and IA2 NSCLC after wedge resection. The 8th edition TNM classification bases the T stage on the size of the invasive component of the tumor. The non-invasive (lepidic) component is not measured in the T category. As such, we thought that the 8th edition TNM staging system would be a better way to determine the indication for wedge resection because it allows for the exclusion of the non-invasive components, which have superior prognoses. Our results suggest that, especially in 8th edition stage IA1 NSCLC, the prognosis after wedge resection is not different from lobectomy. The 5-year DFS rate after wedge resection of 8th edition TNM stage IA1 NSCLC was 91.6%, and the 5-year disease-specific survival rate was 100%. The clinicopathological characteristics were well matched between the lobectomy group and the wedge resection group in stage IA1 NSCLC, and although patients with minimally invasive adenocarcinoma were excluded, the 91.6% 5-year DFS was comparable with other studies in this stage (12). Therefore, we concluded that wedge resection seems to be an acceptable procedure in 8th edition TNM stage IA1 NSCLC.
The 5-year DFS rate was also not statistically different between lobectomy and wedge resection in stage IA2 NSCLC. However, at this stage, the 5-year DFS after wedge resection was 74.0% and the 5-year disease-specific survival was 79.2%, which are not acceptable for stage IA2 NSCLC. Wedge resection itself was not a risk factor for recurrence in univariate analysis in stage IA2 NSCLC, but there were other risk factors related to surgical procedure. For instance, CEA level and lymphovascular invasion, which were both significant risk factors, reflect the aggressiveness of the tumor. In the presence of these risks, wedge resection may be insufficient. Furthermore, both CEA level and lymphovascular invasion have been associated with lymph node upstage after surgery (13-16). It is difficult to dissect N1 nodes when performing wedge resection. As such, the pathologic stage after wedge resection is not accurate. Our multivariate analysis suggested a strong (P=0.053), albeit not statistically significant, association between the number of dissected lymph nodes and recurrence. Since lymph node dissection is rarely performed with wedge resection, this could be interpreted as an increased risk for recurrence of stage IA2 tumors after wedge resection, and in the case of stage IA2 disease, segmentectomy may be a more suitable operation than wedge resection because more lymph nodes can be excised. This should be clarified through further study in the future. On the other hand, our previous research has shown that lymph node upstaging did not occur in clinical N0 NSCLC <1 cm (14,17). Therefore, wedge resection in 8th edition TNM stage IA1 NSCLC with an invasive component size of ≤1 cm is also an acceptable procedure in terms of the possibility of lymph node metastasis.
When performing wedge resection, an adequate resection margin distance is important to achieve complete resection of the tumor (18). According to the National Comprehensive Cancer Network (NCCN) guidelines for NSCLC (Version 2, 2019), the parenchymal resection margins in wedge resection should measure at least 2 cm or be equal to the size of the tumor. In this study, the margin/invasive component ratio was a risk factor for recurrence after wedge resection. In our previous study, adenocarcinoma with a high lepidic component was less likely to recur even if the resection margin was short (19,20). In other words, it appears that the size of the invasive component is more important in determining the resection margin distance than the total tumor size including the lepidic component. Thus, wedge resection should be performed at a distance from the tumor of at least the size of the invasive component.
We evaluated 5-year DFS instead of overall survival because of the high rate of non-cancer related deaths during follow-up among patients with stage I NSCLC (21). DFS is a more accurate measurement for the survival analysis because it reflects the biological behavior of cancer rather than death due to unrelated factors.
This study has a few limitations. First, it was a retrospective review. Second, we obtained data from a single institution, and the sample size was relatively small to generalize our results. However, this study examined data from surgical patients treated by a mainly standardized protocol at our institution, a tertiary hospital in Korea. Furthermore, a very detailed analysis was possible because of the comprehensive information stored in the electronic medical record. We retrieved data that thoroughly described the surgical procedures with lymph node dissection along with exhaustive data from pathologic specimens and pathologic reports. We believe that our data will be useful as the basis for future investigations. A prospective randomized controlled study should be performed to validate our results. Finally, the follow-up period was relatively short. However, recurrence of NSCLC is most commonly reported within a 2-year postoperative period (22), and early recurrence seems to be an accurate reflection of the long-term outcome (23).
In conclusion, the prognosis after wedge resection or lobectomy in stage IA1 NSCLC was not different. Therefore, it seems that wedge resection may be an acceptable procedure for 8th edition TNM stage IA1 NSCLC. In stage IA2 NSCLC, the prognosis was not statistically different between lobectomy and wedge resection, but the 5-year DFS after wedge resection was not acceptable. Therefore, wedge resection can only be conducted in select cases of 8th edition TNM stage IA2 NSCLC, and accurate pathologic staging and tumor type must be considered. The resection margin was the main risk factor for recurrence in both stages for patients undergoing wedge resection. Therefore, when performing wedge resection, the surgeon must ensure that the resection margin distance is longer than the size of the invasive component of the tumor. Further studies that include data from larger cohorts and prospective randomized controlled trials may validate these conclusions and provide more refined results.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital, the Catholic University of Korea (approval ID KC19RESI0064).
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 22-3. [Crossref] [PubMed]
- Taioli E, Yip R, Olkin I, et al. Survival after Sublobar Resection for Early-Stage Lung Cancer: Methodological Obstacles in Comparing the Efficacy to Lobectomy. J Thorac Oncol 2016;11:400-6. [Crossref] [PubMed]
- Cao C, Chandrakumar D, Gupta S, et al. Could less be more?-A systematic review and meta-analysis of sublobar resections versus lobectomy for non-small cell lung cancer according to patient selection. Lung Cancer 2015;89:121-32. [Crossref] [PubMed]
- Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol 2010;5:1583-93. [Crossref] [PubMed]
- Nakamura K, Saji H, Nakajima R, et al. A phase III randomized trial of lobectomy versus limited resection for small-sized peripheral non-small cell lung cancer (JCOG0802/WJOG4607L). Jpn J Clin Oncol 2010;40:271-4. [Crossref] [PubMed]
- Nishio W, Yoshimura M, Maniwa Y, et al. Re-Assessment of Intentional Extended Segmentectomy for Clinical T1aN0 Non-Small Cell Lung Cancer. Ann Thorac Surg 2016;102:1702-10. [Crossref] [PubMed]
- Travis WD, Asamura H, Bankier AA, et al. The IASLC Lung Cancer Staging Project: Proposals for Coding T Categories for Subsolid Nodules and Assessment of Tumor Size in Part-Solid Tumors in the Forthcoming Eighth Edition of the TNM Classification of Lung Cancer. J Thorac Oncol 2016;11:1204-23.
- Rami-Porta R, Asamura H, Travis WD, et al. Lung cancer - major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 2017;67:138-55.
- Rami-Porta R, Eberhardt WEE. Clinical implications of the innovations in the primary tumour and metastasis of the 8(th) edition of the TNM classification for lung cancer. J Thorac Dis 2018;10:S2682-5. [Crossref] [PubMed]
- Goldstraw P, Chansky K, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2016;11:39-51. [Crossref] [PubMed]
- Cho JH, Choi YS, Kim J, et al. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg 2015;99:218-22. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC Lung Cancer Staging Project: Proposals for the Revisions of the T Descriptors in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol 2015;10:990-1003.
- Yamazaki K, Yoshino I, Yohena T, et al. Clinically predictive factors of pathologic upstaging in patients with peripherally located clinical stage IA non-small cell lung cancer. Lung Cancer 2007;55:365-9. [Crossref] [PubMed]
- Moon Y, Kim KS, Lee KY, et al. Clinicopathologic Factors Associated With Occult Lymph Node Metastasis in Patients With Clinically Diagnosed N0 Lung Adenocarcinoma. Ann Thorac Surg 2016;101:1928-35. [Crossref] [PubMed]
- Haruki T, Aokage K, Miyoshi T, et al. Mediastinal nodal involvement in patients with clinical stage I non-small-cell lung cancer: possibility of rational lymph node dissection. J Thorac Oncol 2015;10:930-6. [Crossref] [PubMed]
- Ye B, Cheng M, Li W, et al. Predictive factors for lymph node metastasis in clinical stage IA lung adenocarcinoma. Ann Thorac Surg 2014;98:217-23. [Crossref] [PubMed]
- Moon Y, Park JK, Lee KY, et al. Consolidation/Tumor Ratio on Chest Computed Tomography as Predictor of Postoperative Nodal Upstaging in Clinical T1N0 Lung Cancer. World J Surg 2018;42:2872-8. [Crossref] [PubMed]
- Sawabata N, Ohta M, Matsumura A, et al. Optimal distance of malignant negative margin in excision of nonsmall cell lung cancer: a multicenter prospective study. Ann Thorac Surg 2004;77:415-20. [Crossref] [PubMed]
- Moon Y, Lee KY, Moon SW, et al. Sublobar Resection Margin Width Does Not Affect Recurrence of Clinical N0 Non-small Cell Lung Cancer Presenting as GGO-Predominant Nodule of 3 cm or Less. World J Surg 2017;41:472-9. [Crossref] [PubMed]
- Moon Y, Lee KY, Park JK. Margin Width of Resected Lepidic Lung Cancer Does Not Affect Recurrence After Sublobar Resection. World J Surg 2018;42:1449-57. [Crossref] [PubMed]
- Eguchi T, Kadota K, Park BJ, et al. The new IASLC-ATS-ERS lung adenocarcinoma classification: what the surgeon should know. Semin Thorac Cardiovasc Surg 2014;26:210-22. [Crossref] [PubMed]
- Tremblay L, Deslauriers J. What is the most practical, optimal, and cost effective method for performing follow-up after lung cancer surgery, and by whom should it be done? Thorac Surg Clin 2013;23:429-36. [Crossref] [PubMed]
- Kiankhooy A, Taylor MD, LaPar DJ, et al. Predictors of early recurrence for node-negative t1 to t2b non-small cell lung cancer. Ann Thorac Surg 2014;98:1175-83. [Crossref] [PubMed]