
Comparison of lymphocyte immune phenotypes in bronchoalveolar lavage of non-smoking patients with sarcoidosis and other interstitial lung diseases
Introduction
The increase of CD3+CD4+ T lymphocytes, which are surface markers of T helper 1 and 17 type immune response leading to the granuloma formation, is usually observed in sarcoidosis patients, compared to other interstitial lung diseases (ILDs) (1,2). Nevertheless patients in diverse stages of sarcoidosis, such as patients with Löfgren’s syndrome (LS) and advanced third or fourth chest X-ray (CXR) stage could have completely different BAL cellular profile (3).
CD3+CD4+ T cells are a potential target for therapy. Anti-inflammatory therapies are based on the gene suppression of mediators released by activated CD4+ T cell, such as interleukin-2, tumor necrosis factor-alpha and interferon-gamma. This form of treatment is used in several types of sarcoidosis. However it has been shown that corticosteroid therapy has a serious toxic side effect. Non-steroidal anti-inflammatory drugs have also serious side effects (4). Activated T cells are potential targets for lymphocyte specific treatment in sarcoidosis, however these therapies are still poor (5). Still, there is a need to investigate CD4+ T cells in more detail.
It is important to emphasize that distinct sarcoidosis chest radiographic stages can differ in the composition of the BAL immune cells. Increase of lymphocyte counts with the elevation of CD4+ lymphocyte subset, resulting in the higher CD4+/CD8+ ratio in bronchoalveolar space, is typical for pulmonary sarcoidosis (6-9). Higher CD4+/CD8+ ratio could be observed especially in its acute onset (10). In 80% of sarcoidosis cases bronchoalveolar lavage (BAL) shows a moderate (20–50%) lymphocytosis and CD4+/CD8+ ratio higher than 3.5 in 50% of cases (11,12). Despite favourable outcomes in two-thirds of patients, a third of them experience long-standing disease and are at risk of developing fibrosis (13). In some reports, which contain data about BAL CD4+ and CD8+ lymphocyte subsets, there is no information about sarcoidosis chest radiographic stages or forms of sarcoidosis clinical manifestations (14-16).
Previously only few reports compared BAL cellular profile of different pulmonary sarcoidosis radiographic stages to other interstitial lung diseases, but they do not include information about BAL CD4+ and CD8+ lymphocyte subsets. One of these publications consisted of subjects who were smokers and non-smokers and the second publication do not included information about smoking status of studied subjects (17,18). Smoking status and history should not be omitted (19-22). To avoid the confounding effect of smoking on different BAL lymphocyte subsets we excluded smokers and ex-smokers from our study. We would like to fill a gap in knowledge about differences in lymphocyte subsets between different stages of pulmonary sarcoidosis and other interstitial lung diseases in non-smoking patients.
Methods
Study population
We performed the retrospective analysis of 297 subjects who had interstitial lung disease and were enrolled in the Department of Respiratory Medicine and Tuberculosis, University Hospital Olomouc, the Czech Republic in years 1995–2013. All subjects were non-smokers. All patients gave their informed consent to participate, which was approved by the local Ethical committee of Medical Faculty PU & University Hospital (Olomouc, the Czech Republic). Characteristics of the non-smoking patients with ILDs are provided in Table 1. In to our study were included sarcoidosis patients in early disease stage with/without LS in chest radiographic stage (CXR) ≤1, patients in CXR stage 2 and patients with advanced stage in CXR stage ≥3. Patients with LS have acute and milder form of sarcoidosis with classical symptoms as a fever, erythema nodosum, arthralgia and bilateral hilar lymphadenopathy. Stage 2 is bilateral hilar adenopathy with parenchymal infiltration. Patients in stage 3 have parenchymal infiltration without hilar adenopathy. Stage 4 is characterized by advanced fibrosis (23).

Full table
BAL processing and flow cytometry
Data regarding to the BAL lymphocyte subsets were obtained by routine examination of bronchoalveolar lavage fluid (BALF) for the diagnostic purposes. BALF was collected as it was described elsewhere (24,25). In the years 1995–2004 was used Flow Cytometer Coulter Epics XL and from the year 2004 (Beckman Coulter). From year 2004 was used BD FACSCanto (Becton Dickinson, USA). For Examination of samples with FACSCanto BAL cells were stained with Simultest CD4/CD8 Reagent (342407) (CD4-FITC/CD8-PE) and Simultest CD3/CD19 Reagent (34240) (CD3-FITC/CD19-PE) from BD Biosciences. Differential cell counts were performed routinely in the cytological laboratory of the Department of respiratory medicine using May-Gruenwald-Giemsa stained cytocentrifuge slides of BAL cells.
Statistical analysis
Differences in BAL lymphocyte immune phenotypes among investigated groups were analysed by Mann-Whitney test with SPSS 23. P values of particular comparisons underwent false discovery rate (FDR) correction according to Hochberg and Benjamini to remove false positive results and were marked as Pc, P value after correction (26). The significance level was set at Pc<0.05.
Results
In our analyses, we focused on data regarding to BAL lymphocyte subsets because to our knowledge, there was no report that would provide information about comparison of CD3+, CD3+CD4+, CD3+CD8+ relative numbers and CD3+CD4+/CD3+CD8+ ratio of distinct sarcoidosis stages and other ILD groups, especially in the non-smoking patients. After FDR correction of our data, significant differences were observed between sarcoidosis patients (S) with LS and S CXR ≥3. S patients with LS had elevated relative numbers of CD3+, CD3+CD4+ cells and higher CD3+CD4+/CD3+CD8+ ratio than S CXR ≥3 (Figure 1).
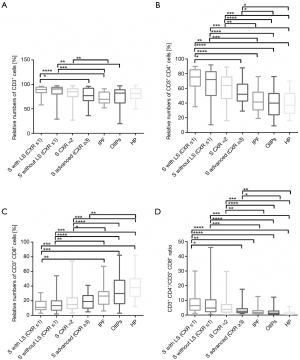
Significant differences in relative numbers of CD3+, CD3+CD4+, CD3+CD8+ and CD3+CD4+/CD3+CD8+ ratio in BAL were noticed between three S groups, S with/without LS CXR ≤1 and S CXR =2, in comparison to the IPF, OIIPs and HP (Figure 1).
Comparison of sarcoidosis patients with advanced sarcoidosis stage, CXR ≥3, to IPF did not show any differences in relative numbers of CD3+, CD3+CD4+, CD3+CD8+ and CD3+CD4+/CD3+CD8+ ratio in BAL. Advanced sarcoidosis showed an increase of CD3+CD4+ relative numbers and CD3+CD4+/CD3+CD8+ ratio in comparison to OIIPs. Advanced sarcoidosis stages showed increased relative numbers of CD3+CD4+, CD3+CD4+/CD3+CD8+ ratio and significant decrease of relative numbers of CD3+CD8+ cells in comparison to patients with HP (Figure 1).
Differential BAL cell counts revealed an increase of relative lymphocyte numbers in all four sarcoidosis groups in comparison to the IPF. Only two groups of sarcoidosis patients, group without LS (CXR ≤1) and CXR stage 2, showed significant increase of relative lymphocyte counts in comparison to OIIPs. Within non-sarcoidosis ILD groups, IPF and OIIPs had lower relative lymphocyte count in comparison to the HP (Figure 2A). Lymphocyte absolute counts did not shown differences between all seven ILD groups (Figure 2B).
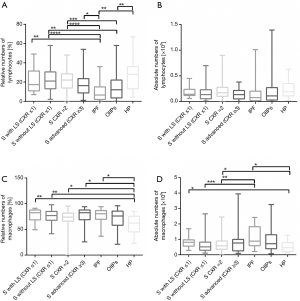
Relative numbers of BAL macrophages showed significant decrease in HP patients in comparison to the all four sarcoidosis groups and IPF (Figure 2C).
Only few differences were observed between non-sarcoidosis ILDs. Differences were noticed between IPF group and HP only in the BAL lymphocyte relative numbers and in macrophage counts (relative and absolute numbers) (Figure 2A,C,D).
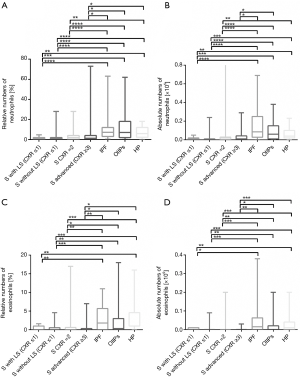
After FDR correction, it became apparent that all sarcoidosis stages showed decreased neutrophils and eosinophils counts in comparison to IPF, OIIPs and HP. However, these BAL cell populations did not show any difference between IPF, OIIPs and HP (Figure 3).
Our analyses comparing smokers and non-smokers or comparing groups of patients with smoking history (smokers plus ex-smokers) and non-smokers were of limited validity because of non-equal numbers of subjects in the compared groups (Table S1). In addition, after FDR correction most of these differences did not attain significance.

Full table
Commenting the data in Table S1, increase of total number of BAL cells (×105), relative and absolute numbers of macrophages in comparison to non-smokers was observed in some ILD groups of smokers. Relative and absolute numbers of lymphocytes were decreased in smokers compared to non-smokers. The analysis did not show differences between smokers and non-smokers in CD3+, CD3+CD4+, CD3+CD8+ and CD3+CD4+/CD3+CD8+ ratio (Table S1).
Analysis of smokers plus ex-smokers in comparison to non-smokers showed fewer differences in differential cell count. However, relative numbers of CD3+CD4+ cells were decreased in smokers plus ex-smokers group when compared to the non-smokers in the group of S without LS and OIIPs (Table S2).

Full table
Discussion
The most pronounced differences in BAL CD3+, CD3+CD4+, CD3+CD8+ lymphocyte subsets and CD3+CD4/CD3+CD8+ ratio were observed when comparing three non-smoking sarcoidosis groups, with/without LS and CXR stage 2, to other ILDs consisting of non-smoking subjects. Less marked differences in BAL lymphocyte subsets and CD3+CD4+/CD3+CD8+ ratio were found between non-smoking sarcoidosis group with advanced disease stage (CXR ≥3), in comparison to other ILDs, consisting of non-smoking subjects.
The increase of CD4+ cells in BAL from sarcoidosis patients with LS resulting in the increased BAL CD4/CD8 ratio is in line with previous findings (3,27,28). Additionally as pulmonary sarcoidosis advances from stage 1 to stage 3 the number of CD4+ cells decreases and the number of CD8+ cells is elevated, resulting in the decrease of the BAL CD4/CD8 ratio (27).
No differences between advanced sarcoidosis stage and IPF group were found in lymphocyte subtypes and this may indicate the change of immunological response towards the fibrotic process which is one of the IPF manifestations. This may show on the similarities between advanced sarcoidosis and IPF. Although there is not enough data about comparison of BAL CD4+ and CD8+ lymphocyte subsets between advanced sarcoidosis and IPF, there are reports that describe histological changes towards fibrotisation in end-stage sarcoidosis. However, exact process of the progression of the granulomatous inflammation to the fibrosis in sarcoidosis remains unsolved (29). It can be hypothesised that there is a shift from pro-inflammatory Th1 to pro-fibrogenic Th2 immune response, which is accompanied by changes of BAL cell subpopulations and inflammatory mediators, fibrogenic and angiogenic factors, as it was described previously in reports comparing sarcoidosis patients in various sarcoidosis stages and IPF (30,31). Nevertheless, there has been a lack of knowledge about differences of BAL immune cells in advanced sarcoidosis and IPF, which could clarify the similarities and differences of these diseases.
We would, therefore, highlight the necessity of the research in the field of advanced sarcoidosis. To our knowledge, only few reports compared the BAL lymphocyte subsets between sarcoidosis and other ILDs. However they did not stratify their patients according to a sarcoidosis stage (14,15,30,32). In particular, Lee et al. [2015] and Capelozzi et al. [2013] found increased relative numbers of CD4+ cells and CD4/CD8 ratio, and decreased relative numbers of CD8+ cells in sarcoidosis groups, without respect to the sarcoidosis stage, in comparison to other ILDs. Their results are in line with our observations in sarcoidosis CXR stage 1 (both with and without LS) and stage 2. However, we did not observe these alterations in advanced sarcoidosis compared to other ILD (15,32). Additionally in report of Capelozzi et al. [2013] sarcoidosis group consisted of only three patients that could be considered as a quite low number of cases for statistical analysis (32). Also Vasakova et al. [2009] reported significantly increased CD4/CD8 ratio in sarcoidosis group, without respect to the sarcoidosis stage, in comparison to the IPF and HP (30). By contrast to the two previously mentioned reports (15,32), Vasakova et al. [2009] provided chest radiographic staging of the patients with sarcoidosis (30).
Regarding the BAL CD3+, CD4+, CD8+ lymphocyte subsets and CD4/CD8 ratio Jara-Palomares et al. [2009] noticed predominantly no differences between sarcoidosis group and other studied groups of ILDs, such as IPF and HP (14), which is in contrast to our results and findings of other authors (15,30,32). Interestingly Jara-Palomares et al. [2009] found differences in BAL CD3+, CD4+, CD8+ lymphocyte subsets and CD4/CD8 ratio only between sarcoidosis group and connective tissue disease (14).
Relative numbers of lymphocytes were increased in all four sarcoidosis groups in comparison to IPF. Only sarcoidosis group without LS in CXR stage ≤1 and CXR stage 2 showed increase of relative lymphocyte counts in comparison to OIIPs. None of these four sarcoidosis stages showed difference in comparison to HP group. These results indicate on lymphocytic cellular pattern which is typical for sarcoidosis and HP (2).
Bronchoalveolar neutrophils and eosinophils represent cells that are also important in diagnosis of ILDs. To our knowledge there is only one study, which focused on comparison of separate sarcoidosis stages to other ILDs (17). However they were interested only in neutrophils and not in eosinophils (17). With the focus on separate sarcoidosis stages the higher proportion of neutrophils in BAL is more specific for sarcoidosis patients in advanced stage (CXR ≥3) as it was observed earlier (17,33).
The increase of neutrophils and eosinophils indicates that these cells could differentiate separate sarcoidosis stages from other ILDs, such as IPF, OIIPs and HP (15). However, in the study of Lee et al. [2015] the difference in neutrophils was found only between sarcoidosis and UIP, not in comparison of sarcoidosis to HP (15), which was also included in our study. Authors did not mention stage of sarcoidosis patients. Previously, eosinophils showed the increase only in eosinophilic pneumonia in comparison to the sarcoidosis, but eosinophilic pneumonia was not subject of our study (15).
Bronchoalveolar macrophages are interesting from the view of the smoking related interstitial lung diseases and they represent BAL cell population that is increased in smokers in comparison to non-smokers (2,22).
The effect of smoking on lymphocyte subsets was noticed previously (21), however in our study we did not found differences in lymphocyte subsets CD3+, CD3+CD4+, CD3+CD8+ and CD3+CD4+/CD3+CD8+ ratio between our smokers and non-smokers in each ILD group. Comparisons of smokers and non-smokers in some ILD groups display increase of total numbers of cells, relative and absolute number of macrophages, and decrease of relative and absolute numbers of lymphocytes in smokers as it was seen previously in sarcoidosis smokers and non-smokers (21).
Smoking group of sarcoidosis patients with CXR stage 2 after extension by ex-smokers showed the increase of CD3+ cells in comparison to the non-smokers. Smokers and ex-smokers in sarcoidosis group without LS had decreased relative numbers of CD3+CD4+ cells in comparison to the non-smokers. The limitation of this analysis was a low number of smokers in each ILD group. Previous report noticed that smoking patients presenting with LS showed increase of relative CD3+ lymphocytes relative numbers and less increased CD4/CD8 ratio in BAL in comparison to the non-smoking patients (21). The increase of CD8+ cells in BAL of the smoking sarcoidosis patients compared to the non-smoking sarcoidosis patients resulted in the significant decrease of CD4/CD8 ratio in smoking sarcoidosis patients (20,21).
Based on our results, BAL cellular components could overlap among diseases and their determination may not be always useful to differentiate between ILDs. The exception is represented by sarcoidosis in early stages, for which increased CD3+CD4+/CD3+CD8+ ratio is characteristic. Importantly, factors influencing BAL cellular profile should be considered, such as smoking or treatment, when interpreting BAL data. Corticosteroid treatment can reduce lymphocytes and its subsets in BALF and thereby decreases the BALF CD4/CD8 ratio (28,34) and should be also considered as possible confounding factor. In this context, usefulness of BAL cellular profile in differential diagnosis of ILDs should be, therefore, carefully reconsidered.
This study has limitations. First, the data used for analyses was collected in long time span in years 1995–2013; from other point of view, this length is a rare situation and may represent an opportunity for long-term follow-up. Second, cell markers were measured by two flow cytometry machines, however their phenotyping results were consistent. Third, only subjects, where data about BAL differential counts and immune phenotype was complete, were chosen for this report, however this stringent approach is methodically correct. Finally, as already mentioned, we were not able to compare non-smoking group to smokers, because smoking cohort contained low numbers of smoking subjects. Despite these limitations, we believe our analyses may complement the current view on BAL lymphocyte subsets and differential cell counts and further, may provide some missing information, especially in non-smoking patients particularly in sarcoidosis.
Conclusions
Our data confirmed the presence of the typical BAL cellular profile in non-smoking patients with sarcoidosis. The BAL cellular profile was helpful namely for differentiation of less advanced sarcoidosis. Its definite diagnostic utility should be the subject of further clinical studies with large numbers of the well characterized patients taking into consideration other clinical factors influencing BAL cellular profile, such as smoking or treatment.
Acknowledgments
We thank to staff of Bronchology Unit at The Department of Respiratory Medicine and Tuberculosis in University hospital Olomouc for routine provision of BAL samples for the diagnostic purposes.
Funding: This work was supported in part by project reg. no. CZ.02.1.01/0.0/0.0/16_019/0000868, LO1304 and IGA_PU_LF_2019_009.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study was approved by the local Ethical committee of Medical Faculty PU & University Hospital (Olomouc, the Czech Republic).
References
- Facco M, Cabrelle A, Teramo A, et al. Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 2011;66:144-50. [Crossref] [PubMed]
- Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guidelines: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004-14. [Crossref] [PubMed]
- Danila E, Norkuniene J, Jurgauskiene L, et al. Diagnostic role of BAL fluid CD4/CD8 ratio in different radiographic and clinical forms of pulmonary sarcoidosis. Clin Respir J 2009;3:214-21. [Crossref] [PubMed]
- Celada LJ, Drake WP. Targeting CD4+ T cells for the treatment of sarcoidosis: a promising strategy? Immunotherapy 2015;7:57-66. [Crossref] [PubMed]
- Patterson KC, Chen ES. The pathogenesis of pulmonary sarcoidosis and implications for treatment. Chest 2018;153:1432-42. [Crossref] [PubMed]
- Crystal RG, Roberts WC, Hunninghake GW, et al. Pulmonary sarcoidosis: A disease characterized and perpetuated by activated lung T-lymphocytes. Ann Int Med 1981;94:73-94. [Crossref] [PubMed]
- Hunninghake GW, Crystal RG. Pulmonary sarcoidosis: a disorder mediated by excess helper T-lymphocyte activity at sites of disease activity. N Engl J Med 1981:429-34. [PubMed]
- Robinson BW, Rose AH, Thompson PJ, et al. Comparison of bronchoalveolar lavage helper/suppressor T-cell ratios in sarcoidosis versus other interstitial lung diseases. Aust N Z J Med 1987;17:9-15. [Crossref] [PubMed]
- Petrek M, Kolek V. Determination of T-lymphocyte subsets in bronchoalveolar lavage of patients with pulmonary sarcoidosis. Acta Univ Palacki Olomuc Fac Med 1991;130:169-77. [PubMed]
- Ward K, O'Connor C, Odlum C, et al. Prognostic value of bronchoalveolar lavage in sarcoidosis: the critical influence of disease presentation. Thorax 1989;44:6-12. [Crossref] [PubMed]
- Valeyre D, Prasse A, Nunes H, et al. Sarcoidosis. Lancet 2014;383:1155-67. [Crossref] [PubMed]
- Spagnolo P, Rossi G, Trisolini R, et al. Pulmonary sarcoidosis. Lancet Respir Med 2018;6:389-402. [Crossref] [PubMed]
- Bonham CA, Strek ME, Patterson KC. From granuloma to fibrosis: sarcoidosis associated pulmonary fibrosis. Curr Opin Pulm Med 2016;22:484-91. [Crossref] [PubMed]
- Jara-Palomares L, Martin-Juan J, Gomez-Izquierdo L, et al. Bronchoalveolar lavage findings in patients with diffuse interstitial lung disease: prospective study of a cohort of 562 patients. Arch Bronconeumol 2009;45:111-7. [Crossref] [PubMed]
- Lee W, Chung WS, Hong KS, et al. Clinical usefulness of bronchoalveolar lavage cellular analysis and lymphocyte subsets in diffuse interstitial lung diseases. Ann Lab Med 2015;35:220-5. [Crossref] [PubMed]
- Tanriverdi H, Erboy F, Altinsoy B, et al. Bronchoalveolar lavage fluid characteristics of patients with sarcoidosis and non-sarcoidosis interstitial lung diseases: Ten-year experience of a single center in Turkey. Iran Red Crescent Med J 2015;17:e31103. [Crossref] [PubMed]
- Roth C, Huchon GJ, Arnoux A, et al. Bronchoalveolar cells in advanced pulmonary sarcoidosis. Am Rev Respir Dis 1981;124:9-12. [PubMed]
- Kucejko W, Chyczewska E, Naumnik W, et al. Concentration of surfactant protein D, Clara cell protein CC-16 and IL-10 in bronchoalveolar lavage (BAL) in patients with sarcoidosis, hypersensitivity pneumonitis and idiopathic pulmonary fibrosis. Folia Histochem Cytobiol 2009;47:225-30. [Crossref] [PubMed]
- Watters LC, King TE, Cherniack RM, et al. Bronchoalveolar lavage fluid neutrophils increase after corticosteroid therapy in smokers with idiopathic pulmonary fibrosis. Am Rev Respir Dis 1986;133:104-9. [Crossref] [PubMed]
- Valeyre D, Soler P, Clerici C, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax 1988;43:516-24. [Crossref] [PubMed]
- Drent M, van Velzen-Blad H, Diamant M, et al. Relationship between presentation of sarcoidosis and T lymphocyte profile. A study in bronchoalveolar lavage fluid. Chest 1993;104:795-800. [Crossref] [PubMed]
- Karimi R, Tornling G, Grunewald J, et al. Cell recovery in bronchoalveolar lavage fluid in smokers is dependent on cumulative smoking history. PLoS One 2012;7:e34232. [Crossref] [PubMed]
- Hunninghake GW, Costabel U, Ando M, et al. Statement on Sarcoidosis. Am J Respir Crit Care Med 1999;160:736-55. [Crossref] [PubMed]
- Petrek M, Kolek V. T-lymphocyte subpopulations in bronchoalveolar lavage in pulmonary sarcoidosis and in other diseases of the pulmonary interstitium. Cas Lek Cesk 1993;132:365-8. [PubMed]
- Petrek M, Gibejova A, Drabek J, et al. CC chemokine receptor 5 (CCR5) mRNA expression in pulmonary sarcoidosis. Immunol Lett 2002;80:189-93. [Crossref] [PubMed]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 1995;57:289-300.
- Danila E, Jurgauskiene L, Malickaite R. BAL fluid cells and pulmonary function in different radiographic stages of newly diagnosed sarcoidosis. Adv Med Sci 2008;53:228-33. [Crossref] [PubMed]
- Danila E, Jurgauskiene L, Norkuniene J, et al. BAL fluid cells in newly diagnosed pulmonary sarcoidosis with different clinical activity. Ups J Med Sci 2009;114:26-31. [Crossref] [PubMed]
- Shigemitsu H, Azuma A. Sarcoidosis and interstitial pulmonary fibrosis; two distinct disorders or two ends of the same spectrum. Curr Opin Pulm Med 2011;17:303-7. [Crossref] [PubMed]
- Vasakova M, Sterclova M, Kolesar L, et al. Bronchoalveolar lavage fluid cellular characteristics, functional parameters and cytokine and chemokine levels in interstitial lung diseases. Scand J Immunol 2009;69:268-74. [Crossref] [PubMed]
- Antoniou KM, Soufla G, Proklou A, et al. Different activity of the biological axis VEGF-Flt-1 (fms-like tyrosine kinase 1) and CXC chemokines between pulmonary sarcoidosis and idiopathic pulmonary fibrosis: a bronchoalveolar lavage study. Clin Dev Immunol 2009;2009:537929. [Crossref] [PubMed]
- Capelozzi VL, Faludi EP, Balthazar AB, et al. Bronchoalveolar lavage improves diagnostic accuracy in patients with diffuse lung disease. Diagn Cytopathol 2013;41:1-8. [Crossref] [PubMed]
- Ziegenhagen MW, Rothe ME, Schlaak M, et al. Bronchoalveolar and serological parameters reflecting the severity of sarcoidosis. Eur Respir J 2003;21:407-13. [Crossref] [PubMed]
- Erkkilä S, Fröseth B, Hellström PE, et al. Inhaled budesonide influences cellular and biochemical abnormalities in pulmonary sarcoidosis. Sarcoidosis 1988;5:106-10. [PubMed]


