
Chinese guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia in adults (2018 Edition)
Hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP) are the most common hospital-acquired infections in China. The difficulty in diagnosis and treatment of HAP/VAP leads to high mortality. In China, it has been nearly two decades since the initial “Guideline for the Diagnosis and Treatment of Hospital-acquired Pneumonia (draft)” was published in 1999 (1). Afterwards, a number of guidelines for HAP/VAP have been published or updated at home and abroad (2-10). The definitions of HAP/VAP have been changing as more and more relevant researches available highlighting greater details. Moreover, there are growing evidence in the epidemiology, etiology, clinical diagnosis and treatment of HAP/VAP, especially the accumulating evidence from the researches in China, which shows that the distribution and antibiotic resistance rate of HAP/VAP pathogens in China are largely different from the data reported in other countries. Therefore, it is necessary to amend the initial guideline in 1999 accordingly in order to better guide clinical practice.
The initial HAP guideline was updated by the Infection Study Group of Chinese Thoracic Society, Chinese Medical Association (CMA). The overall framework and main contents of the updated HAP/VAP guideline were finalized after multiple rounds of face-to-face workshops. After repeated discussions among all the members of the Infection Study Group and extensive consultations from domestic and foreign experts in related fields, the draft was revised several times. Finally, the consensus was reached on the basis of evidence-based medicine. The level of evidence and grading of recommendation are defined the same way as in “Diagnosis and treatment of community-acquired pneumonia in adults: 2016 clinical practice guidelines by the Chinese Thoracic Society, Chinese Medical Association (2016 Edition)” (11). The grading system classifies recommendations according to the balance of the benefits and downsides (harms, burden, and cost) on the basis of the quality of evidence. The quality of evidence reflects the confidence in estimates of the true effects of an intervention (Table 1). In general, the higher the quality of evidence is, the stronger the grade of recommendation. However, they do not fully correspond to each other. The source of evidence, willingness and values of patients, as well as resource consumption should also be considered when making a recommendation. We emphasize that under the premise of the same level of evidence, the evidence and research results in China should be adopted preferentially.

Full table
This guideline applies to the immunocompetent HAP/VAP patients aged 18 years or older. The main body of this document is composed of 8 sections and 1 annex. It is expected that the revision and popularization of this HAP/VAP guideline will further standardize the diagnosis and treatment of HAP/VAP in China.
Definitions
HAP is defined as a pneumonia not incubating at the time of hospital admission and occurring 48 hours or more after admission in patients not receiving invasive mechanical ventilation during hospitalization. VAP is defined as a pneumonia occurring >48 hours after endotracheal intubation or tracheotomy to receive mechanical ventilation. A pneumonia occurring within 48 h after removing mechanical ventilation and extubation is also considered as VAP (2,3).
At early times, HAP was defined as any parenchymal lung infection occurring in hospital due to the pathogens existing in the hospital environment. In the Chinese “Guideline for the Diagnosis and Treatment of Hospital-acquired Pneumonia (draft)” published in 1999, the definition of HAP covered the pneumonia occurring after establishing artificial airway and mechanical ventilation (1). There are various confounding factors in the numerous HAP clinical studies previously conducted at home and abroad, including some patients undergoing mechanical ventilation. However, it is generally agreed that VAP is a special type of HAP. Considering the significant difference between HAP and VAP in terms of clinical features, empiric treatment, and prevention strategy, the Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia issued by the Infectious Diseases Society of America/American Thoracic Society (IDSA/ATS) in 2005 (2005 IDSA/ATS HAP/VAP guideline) have classified HAP into HAP in narrow sense and VAP (2). The evidence in recent years further confirms that HAP is considerably different from VAP in empiric treatment and clinical prognosis. The updated IDSA/ATS HAP/VAP guideline in 2016 specifically emphasizes that HAP only refers to the pneumonia occurring after hospital admission in the patients without endotracheal intubation and not associated with mechanical ventilation, while VAP represents the pneumonia occurring after endotracheal intubation and mechanical ventilation. HAP population is therefore totally different from VAP population (7). Nevertheless, there are geographic difference and different understandings about HAP/VAP. Currently, controversies exist among European and American countries regarding the definition of HAP/VAP. We still think that VAP is a special type of HAP. This Guideline will address HAP and VAP separately in sections of etiology, treatment and prevention due to the particularity of VAP. The HAP in patients who have to receive endotracheal intubation and mechanical ventilation due to disease progression still belongs to HAP, but such cases will be managed in the same way as VAP. A pneumonia occurring in the inpatients receiving non-invasive ventilation still belongs to narrowly defined HAP.
Epidemiology
HAP/VAP belongs to hospital-acquired infection. Large scale cross section survey of nosocomial infections in China showed that the incidence of hospital-acquired infection ranged from 3.22% to 5.22% in hospitalized patients, and the incidence of hospital-acquired lower respiratory tract infection was 1.76% to 1.94% (12,13). In the US, the incidence of hospital-acquired infection is 4.0% in inpatients, of which pneumonia accounts for 21.8% (14). The researches in China and other countries have demonstrated that lower respiratory tract infection, including HAP/VAP, accounts for the largest proportion of hospital-acquired infections.
The results of studies in other countries indicated that the incidence of HAP ranged from 5 to 10 cases per 1,000 hospitalized patients, and HAP accounted for 25.0% of the total number of infections in intensive care unit (ICU). HAP increases the average length of hospital stay by 7–10 days (15), and dramatically increases the in-hospital medical cost. HAP is also an important direct cause of death in critically ill patients, specifically associated with mortality of 15.5–38.2% (16,17).
A clinical HAP survey conducted in 13 large teaching hospitals in China has reported that the average incidence of HAP is 1.4% in Department of Respiratory Medicine and respiratory ICU (RICU) combined, specifically 15.3% in RICU and 0.9% in general wards. The all-cause mortality of HAP is 22.3% and 34.5% for VAP. HAP is associated with 23.8±20.5 days of hospital stay on average, 10 days longer than that of non-HAP patients. For HAP patients, the mean duration of antimicrobial therapy is 19±17 days. The in-hospital medical cost per HAP patient is at least 90,000 Yuan ($13,600) higher than that for a non-HAP inpatient, of which more than 66,000 Yuan ($10,000) is incurred after HAP. Every HAP patient spends up to 27,000 Yuan ($4,100) on antimicrobial agents (18).
Large scale studies worldwide have shown that the incidence of VAP is 2.5–40.0% (or 1.3 to 20.2 cases per 1,000 mechanical ventilation days) in ICU patients, associated with mortality of 13.0–25.2% (19-21). A survey conducted in China reports that among the 17,358 patients in ICU in 46 hospitals, the total days of endotracheal intubation is 91,448. The incidence of VAP is 8.9 cases per 1,000 mechanical ventilation days (22). The incidence of VAP is 9.7–48.4% (or 1.3 to 28.9 cases per 1,000 mechanical ventilation days in the patients receiving mechanical ventilation, associated with mortality of 21.2–43.2% (18,23-27). The results of relevant studies both at home and abroad indicate that the attributable mortality rate is up to 38.9–60.0% if VAP is caused by multi-drug resistant (MDR) or pan-drug resistant (PDR) pathogens. The mortality of VAP is associated with age, comorbidity diabetes mellitus or chronic obstructive pulmonary disease (COPD), septic shock, and highly resistant pathogens (27-31). VAP results in 5.4–21.8 days longer of mechanical ventilation, 6.1–20.5 days longer of ICU stay, and 11.0–32.6 days longer of hospital stay. In the US, VAP increases the hospital cost by $40,000 in each case on average (20,32-34). These clinical data are provided for reference only because the diagnostic criteria, study protocol, study subjects, and statistical method are inconsistent across the studies, and therefore the reported incidence and mortality of HAP/VAP vary greatly (15-36).
Risk factors and pathogenesis
Risk factors
The risk factors for incidence of HAP/VAP involve many aspects, which can be classified into patient-related factors and treatment-related factors as shown in Table 2 (18,25,37-39). A patient usually has multiple risk factors simultaneously or other confounding factors, contributing to the occurrence and progression of HAP/VAP. For this reason, it is important to manage the underlying diseases, and strengthen appropriate infection prevention and control measures.

Full table
Pathogenesis
HAP and VAP share the common pathogenesis that the responsible pathogen reaches the distal end of bronchi and alveoli, breaks through the defense mechanisms of the host, colonizes and multiplies in lung tissues, which causes invasive damage. Pathogenic microorganisms gain access to lower respiratory tract primarily via the following two ways: (I) aspiration: exposure to risk factors such as antimicrobial agents, antacids or indwelling gastric tube may change the normal oral flora of inpatients. The oropharyngeal secretion containing potentially pathogenic bacteria can enter lower respiratory tract through epiglottis or endotracheal intubation. This is the main route of infection caused by endogenous pathogenic microorganisms (38,39); (II) inhalation: the pathogenic microorganisms enter lower respiratory tract by inhalation in the form of aerosol or hydrogel microparticles. This is also an important cause resulting in outbreak of nosocomial infection. The inhaled pathogenic microorganisms are usually exogenous pathogens, such as Mycobacterium tuberculosis, Aspergillus and viruses. Additionally, HAP/VAP may result from other route of infection, for example, dissemination of the responsible pathogen from blood stream to lungs, direct dissemination from adjacent tissues or infection due to contaminated medical devices.
The mechanism of VAP is slightly different from HAP in that endotracheal intubation makes the relatively bacteria-free lower respiratory tract expose directly to external environment, and therefore increases the difficulty of oral hygiene. The bacteria colonizing the oropharynx proliferate massively. The oral secretion containing massive amount of bacteria enters lower respiratory tract through the gap between balloon and tracheal wall in the presence of various factors (balloon deflation or underpressure, change of position) (40). The presence of endotracheal intubation makes the patient unable to cough effectively, which interferes with the function of mucociliary clearance, and reduces the protective ability of airway, and hence increases the risk of developing VAP. Biofilm may readily form on the internal and external surfaces of the tracheal cannula. Many factors (aspiration of sputum, for example) can cause exfoliation of the generated biofilm, which may obstruct small airways, and then lead to VAP (41). In addition, analgesic agents and sedatives are usually used to alleviate patient’s intolerance to endotracheal intubation. Such drugs will suppress the ability of the patient to cough, and so increase the risk of VAP (42).
HAP/VAP may progress gradually from localized infection to sepsis, or even septic shock. The main mechanism underlying this process is that the pathogenic microorganisms enter blood and induce systemic inflammation out of control, which results in multiple organ dysfunction (including respiratory system, as well as circulatory, urinary, nervous, and coagulation systems), and metabolic disorder (43,44).
Etiology
The HAP/VAP in immunocompetent patients is usually caused by bacteria, and infrequently by viruses or fungi. The distribution and antibiotic resistance profile of the common pathogens vary with geographic region, level of hospital, patient population and the extent of antibiotic exposure, and may change over time. The common pathogens of HAP/VAP in China include Acinetobacter baumannii, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, and Escherichia coli. It is more important to know the data of antimicrobial resistance surveillance in local hospitals. Empiric antimicrobial therapy should be based on the latest antimicrobial susceptibility data of the pathogens isolated from the region, the hospital, or even the specific department.
Pathogenic spectrum
Unlike the US and European countries, few large-scale epidemiological studies are available on HAP in China. Three survey studies on HAP etiology conducted in large general hospitals have shown that the pathogenic spectrum of HAP in China is highly different from that in the US and European countries, mainly evidenced by the proportion of the most common bacterial pathogens, including A. baumannii (16.2–35.8%), P. aeruginosa (16.9–22.0%), S. aureus (8.9–16.0%), and K. pneumoniae (8.3–15.4%) (28). The proportion of P. aeruginosa and A. baumannii in secondary hospitals is slightly lower than that in tertiary hospitals, while K. pneumoniae shows higher proportion in secondary hospitals than in tertiary hospitals (18,45,46). About 70% of the HAP patients were old people (≥65 years of age). As for these elderly patients, P. aeruginosa accounts for higher percentage, and A. baumannii relatively lower percentage (18,45,47,48) (Table 3).

Full table
VAP patients in China are mostly identified in ICU. The pathogenic spectrum of VAP is slightly different from that of HAP, evidenced by even higher proportion of A. baumannii (35.7–50.0%), followed by similar proportion of P. aeruginosa and S. aureus (Table 4). In addition, the proportion of P. aeruginosa is also higher in the elderly patients with VAP (≥65 years) than in other patient populations (18,49-54).

Full table
Currently, no high quality, prospective epidemiological study is conducted on HAP/VAP in secondary or lower level hospitals in China. Majority of the reports currently available are retrospective. The above data are provided for reference only.
Antibiotic resistance of common pathogens
Antimicrobial resistance poses a serious challenge to the treatment of HAP/VAP. In clinical practice, MDR is defined as resistance to 3 or more classes of antimicrobial agents (excluding constitutive resistance), while extensive drug resistance (XDR) is defined as resistance to all antimicrobial agents except only 1 to 2 classes. Pan-drug resistance (PDR) means that a pathogen is resistant to all the accessible antimicrobial agents and those available in the panel of antimicrobial agents included in routine susceptibility testing.
The common antibiotic-resistant bacteria identified in HAP/VAP patients include carbapenem-resistant A. baumannii (CRAB), carbapenem-resistant P. aeruginosa (CRPA), extended-spectrum β-lactamases (ESBLs)-producing Enterobacteriaceae, methicillin-resistant S. aureus (MRSA), and carbapenem-resistant Enterobacteriaceae (CRE), etc. According to the multi-center, bacterial resistance surveillance networks in China, i.e., China Surveillance Network for Bacterial Resistance (CHINET) and Chinese Antimicrobial Resistance Surveillance of Nosocomial Infection (CARES), the prevalence of CRAB is up to 60–70% in the strains isolated from various clinical specimens including blood, urine and sputum, while the corresponding prevalence of CRPA is 20–40%, and the prevalence of ESBLs-producing K. pneumoniae and E. coli is 25–35% and 45–60%, respectively. The prevalence of MRSA is 35–40% in the S. aureus isolates, and that of CRE is 5–18% in the Enterobacteriaceae isolates (55,56). Some antibiotic-resistant bacteria such as MRSA, show higher prevalence in the strains isolated from sputum.
The CARES data on antibiotic resistance in HAP/VAP pathogens during 2007–2013 indicated that the prevalence of MDR A. baumannii increased over time, while the prevalence of MDR P. aeruginosa decreased, from 23% in 2007 to 10.3% in 2013. The prevalence of MDR strains in VAP is generally higher than that in HAP except CRE strains (0.7% in VAP, 1.9% in HAP), specifically, CRAB (63.9%, 59.8%), CRPA (41.0%, 33.4%), ESBLs-producing E. coli (64.7%, 57.3%), ESBLs-producing K. pneumoniae (47.4%, 32.4%), and MRSA (85.7%, 74.3%). The prevalence of CRE, especially carbapenem-resistant K. pneumoniae (CRKP), is increasing. The data from China Antimicrobial Resistance Surveillance System (CARSS) in 2015 showed that the prevalence of CRKP was 4.9% and CRAB 52.1% in the corresponding pathogens isolated from lower respiratory tract in the Department of Respiratory Medicine in China. The prevalence of CRKP and CRAB was 5.2% and 53.5% in tertiary hospitals, higher than that in secondary hospitals (2.5% and 33.9% respectively). The prevalence of CRKP and CRAB in RICU is higher than that in general wards. The prevalence of ESBLs-producing Enterobacteriaceae, especially ESBLs-producing E. coli, in secondary hospitals is similar to or even higher than that in tertiary hospitals (63.9% versus 53.5%) (57).
The data from CHINET and CARES indicate that polymyxin B (97–100%) and tigecycline (85–100%) inhibit the highest percentage of A. baumannii isolates. More than 70% of the P. aeruginosa strains are still susceptible to polymyxin, amikacin, piperacillin/tazobactam, cefepime, ciprofloxacin, ceftazidime, meropenem, and imipenem. E. coli and K. pneumoniae are highly susceptible to carbapenems (82–98%), β-lactam/β-lactamase inhibitor combinations (80–96%), and amikacin (90–97%). S. maltophilia isolates are highly susceptible to minocycline (81–94%), levofloxacin (76–90%), and trimethoprim/sulfamethoxazole (67–92%). Vancomycin, teicoplanin, and linezolid remain highly active against MRSA strains (100% susceptible).
It should be noted that both the distribution and antibiotic resistance profile of HAP/VAP pathogens are very different between large (tertiary) hospitals and primary/secondary hospitals in urban area of China. The data from relevant high-quality studies in primary care hospitals are still lacking severely. Therefore, the empiric antimicrobial regimen prescribed in primary care hospitals should be based on local microbiological data as much as possible, rather than completely depending on the data from large urban hospitals.
Diagnosis and differential diagnosis
Clinical diagnostic criteria
HAP/VAP varies in clinical manifestation and disease severity, which may develop rapidly from uncomplicated typical pneumonia to severe pneumonia complicated with sepsis, or even septic shock. Currently, no single “gold standard” is available for clinical diagnosis of HAP/VAP. The clinical diagnosis of pneumonia is more accurate when more of the following relevant clinical conditions are satisfied.
Clinical diagnosis can be established when a new or progressive infiltrate, consolidation, or ground glass opacity is revealed on chest radiograph or CT scan (58,59), plus 2 or more of the following 3 criteria: (I) fever >38 °C; (II) purulent airway secretions; (III) peripheral white blood cell count >10×109/L or 9/L (7).
Radiology is essential and important for diagnosing HAP/VAP. Chest X-ray film should be taken routinely, and chest CT scan should be considered if possible. For critically ill patients or other patients who are unable to receive chest CT scan, point-of-care lung ultrasound can be considered if possible (60,61). Lung ultrasound, performed by an experienced physician, is helpful to monitor lung aeration changes and differentiate pneumonia from pulmonary embolism, atelectasis, or other pulmonary diseases (62) (IB). In the process of clinical decision-making, one or more imaging techniques may be ordered when clinically indicated in order to improve the rate of early diagnosis.
Etiological diagnosis
The pathogenic microorganism can be identified on the basis of clinical diagnosis if any of the following condition is satisfied.
- Pathogen isolated from qualified sample of lower respiratory tract secretion (neutrophils >25 cells per low power field, epithelial cells <10 per low power field, or the ratio between neutrophils and epithelial cells >2.5:1), protected specimen brush (PSB), bronchoalveolar lavage fluid (BALF), lung tissues or sterile body fluid, AND consistent with clinical manifestations (5).
- Fungi presented in pathological, cytopathological or direct microscopic examination of lung tissues, associated with relevant evidence of tissue damage (63,64).
- Seroconversion is observed for IgM antibody against atypical pathogen or virus or a 4-fold or greater rise in specific IgG antibody titer between acute and convalescent sera. Viral antigen assay, nucleic acid test, or culture with respiratory tract secretion is positive for the target virus during epidemic of respiratory viruses, associated with a history of epidemiological exposure (5).
Differential diagnosis
HAP/VAP is not specific in clinical manifestation and imaging results, which should be differentiated from other febrile diseases with concomitant pulmonary opacity, including infectious and non-infectious diseases (65).
- Other infectious diseases affecting lungs: (i) systemic infection involving lungs, for instance: catheter-related bloodstream infection, infective endocarditis, which may lead to multiple secondary pulmonary abscesses; (ii) focal infection involving lungs such as subdiaphragmatic abscess, liver abscess. The key points for differentiation include detailed history inquiry and physical examination, identification of extra-pulmonary site of infection, and pathogen-specific tests.
- Common non-infectious diseases easily confused with HAP: (i) acute pulmonary thromboembolism accompanied by pulmonary infarction (66); (ii) atelectasis; (iii) acute respiratory distress syndrome (ARDS) (67); (iv) pulmonary edema (68); (v) Other diseases, such as tumor, bronchiectasis, drug-induced lung disease, connective tissue disease, and neurogenic fever. The differentiation is primarily based on how well the underlying diseases are controlled, and meanwhile infective fever should be excluded.
Utility of laboratory techniques in the diagnosis and treatment of HAP/VAP
After clinical diagnosis of HAP/VAP is established, specimens should be collected actively for microbiological tests.
Collection of specimens: including the specimens from respiratory tract, blood, and pleural effusion
Respiratory tract specimens: mainly include sputum (airway aspirate), BALF, and lung tissue. The specimens should be firstly subjected to smear and microscopic examination as soon as possible (e.g., gram stain, acid-fast stain, as well as potassium hydroxide wet mounts preparation and methenamine silver stain if necessary), followed by culture, antigen assay, and nucleic acid quantification, etc. (IIIC).
Respiratory tract specimens can be obtained by non-invasive or invasive approaches. Non-invasive approach means collection of the following specimens, including coughed-up phlegm, nasopharyngeal swab, nasopharyngeal aspirates or endotracheal aspiration (ETA). Invasive approach refers to the fact that specimens (BALF or tissue samples) are obtained from the lower respiratory tract via a bronchoscope or percutaneous lung biopsy. Invasive sampling with quantitative cultures generally does not show advantages over non-invasive sampling with semiquantitative cultures in predicting the prognosis (69,70). Quantitative culture of airway secretions is a highly demanding technique and may not be able to improve patient outcome. It should be used only when necessary and accessible. For HAP patients, it is recommended to use non-invasive method to obtain the initial respiratory tract specimens for smear and semi-quantitative cultures. Invasive sampling with microbiological testing is necessary in patients failed to respond to empiric treatment or suspected to be infected by special pathogens, or routine respiratory tract culture failed to identify the pathogens (IIIB). ETA could be routinely sampled in VAP patients. Additionally, it is also convenient to collect invasive sample of respiratory tract for smear and semiquantitative cultures to identify the pathogen via artificial airway in VAP patients. Culture of airway secretions twice a week is helpful in predicting the etiology of VAP (71). The conversion from positive to negative result of quantitative culture is helpful for clinicians to determine whether it is appropriate to discontinue antimicrobial agents (72,73) (IIB).
Blood: blood culture is an important way for diagnosing bacteremia. For adults, 2 or 3 sets of blood samples should be collected from different body sites each time for blood culture. Blood samples (one set) collected from the same puncture site are usually injected into an aerobic bottle and an anaerobic bottle concurrently. And 8–10 mL of blood sample is needed in each bottle in order to increase positivity (58). Blood samples should be drawn at the onset of chills or initial fever, preferably before the use of antimicrobial agents (74).
Pleural effusion: thoracentetis is recommended for HAP/VAP patients with pleural effusion for routine tests, biochemical tests, smear (gram stain, acid-fast stain), and culture of microorganisms.
Interpretation of etiological testing results: including smear and microscopic examination, microbiological culture, antigen test, high throughput sequencing, and other molecular biological techniques
Smear/microscopic examination: smear and gram stain of ETA samples showing ≥2% of the leucocytes contain intracellular organisms per high-power field is valuable for etiological diagnosis of VAP patients (75), and is useful for the initial empiric antimicrobial therapy (76-81).
Microbiological culture: conventionally, the microorganism is highly suspected to be the culprit pathogen if it meets any of following conditions: ≥107 cfu/mL of bacteria in quantitative sputum culture, ≥105 cfu/mL in ETA microbiological culture, ≥104 cfu/mL in BALF quantitative culture, ≥103 cfu/mL in PSB sampl culture (82-84). Acinetobacter spp., Pseudomonas spp., and Candida may colonize the native and/or artificial airway in patients receiving mechanical ventilation. Such microorganisms revealed by culture should be clarified whether they are pathogenic microorganisms or not. It is recommended to evaluate the role of these microorganisms comprehensively in terms of the following three factors: (I) host: including immune status, underlying diseases and the current clinical manifestations; (II) bacteria: such as whether there are leucocytes containing intracellular organisms in smear/microscopic examination of airway secretions, whether the result of smear/microscopic examination is consistent with microbiological culture findings or not, and how many bacteria colonies there are; (III) antimicrobial agent: for instance, recent use of antimicrobial agents, whether clinical symptoms improve after antimicrobial therapy against target pathogen. Microorganisms identified from airway secretions could be considered as colonization or contamination if the patient does not have pneumonia-related clinical manifestations or laboratory results. Blood culture is important for early diagnosis of infection and pathogen-specific antimicrobial therapy. However, conclusion that microorganism originates from lungs cannot be made from a positive blood culture, because lung is the source of infection in only 10–37% of bacteremic cases (83,85-89). Positive result of pleural effusion culture is helpful for clarifying etiological diagnosis, especially when the specimen is obtained by thoracentesis or at the time of initial placement of catheter in thoracic cavity. The positive result of the specimen directly sampled from the indwelling catheter should be interpreted cautiously because of the possibility of contamination (IIIC). Positive result of respiratory viruses culture confirms viral infection.
Pathogen-specific antigen test: urinary antigen detection targeting S. pneumoniae and Legionella pneumophila, and detection of serum cryptococcal capsular polysaccharide antigen are highly sensitive and specific. Two consecutive positive results of serum 1,3-β-D-glucan assay (G test), serum or BALF galactomannan (GM) antigen test (only once in case of BALF) support the diagnosis of invasive fungal infection.
High throughput sequencing and other molecular biological techniques: clinical metagenomics based on sequencing technique can help determine the potential pathogen by analyzing the content or abundance of microorganic DNA or RNA in clinical specimens. This technique can significantly increase the sensitivity of etiological test, and shorten the turnaround time of laboratory test. It is especially useful for the diagnosis of infections caused by pathogens rarely encountered. This technique can be used judiciously to identify pathogens which cannot be identified by established methods, or in patients who fail to respond to appropriate and standard anti-infective treatment. However, the results should be evaluated comprehensively in combination with epidemiological data and clinical features to determine whether it is true pathogen. There are many challenges in clinical application of this technique, including interference by human genome in specimen, bioinformatics analysis, result assessment and interpretation. In particular, respiratory tract is not germ-free, so the presence of the nucleic acid derived from a large number of colonizing bacteria poses challenge to clinical interpretation of testing results (90-92).
Infection-related biomarkers
Both C-reactive protein (CRP) and procalcitonin (PCT) are most commonly used biomarkers in clinical practice to determine the presence or absence of infection (93,94). CRP increases remarkably in case of infection, but with low specificity in detecting infection (95,96). It can be used as adjunctive test to support diagnosis (IIC). PCT responds quickly to bacterial infection and sepsis (97,98). It is a more specific biomarker of bacterial infection, when compared to CRP (93,99). Higher PCT value indicates more severe bacterial infection, and higher possibility of bacterial VAP and sepsis (97,100). The diagnostic efficiency of PCT is affected by prior exposure to antimicrobial agents, but not affected by the type of disease or onset time of VAP. Furthermore, PCT is also an important predictor of VAP mortality (100). Dynamic monitoring of PCT level in the course of disease is helpful for deciding the duration of antimicrobial treatment (101-104) (IIB). It should be emphasized that neither CRP nor PCT can replace microbiological testing. Any biomarker of infection should be combined with clinical manifestations to make comprehensive judgement. The dynamic change of biomarkers is usually more valuable than the absolute value (94). Early empiric antimicrobial therapy should not be delayed for waiting for the results of laboratory testing in order to increase treatment success rate.
Evaluation of disease severity
The severity evaluation of HAP/VAP is important for empiric selection of antimicrobial agents and prognosis estimation, but there is not a unified criterion currently. The commonly used scoring systems for evaluating disease severity include sequential organ failure assessment (SOFA) (Table 5) and acute physiology and chronic health evaluation (APACHE-II) score. These scoring systems are equal in predicting mortality. Mortality rate increases with the scores (105). SOFA score focuses on the evaluation of organ dysfunction or failure. It is associated with the relapse of VAP (106,107). While, APACHE-II score >16 is an independent predictor of death in VAP patients (106-109). Some scholars suggest that SOFA score can be used as a criterion to assess the severity of disease. For non-ICU patients, quick SOFA (qSOFA) score is simpler, more convenient and efficient than SOFA score in predicting in-hospital mortality (110). qSOFA score is composed of altered mental status, systolic blood pressure ≤100 mmHg (1 mmHg =0.133 kPa), and respiratory rate ≥22 breaths/min. Clinicians should alert the occurrence of critical illness when qSOFA score ≥2.

Full table
In this guideline, HAP patients are considered critically ill and at high risk of death if satisfying any of the following criteria: (I) requiring endotracheal intubation and mechanical ventilation; (II) septic shock still requiring vasoactive agents after active fluid resuscitation. Unlike the narrowly defined HAP, VAP should be generally considered as critical illness. However, in some cases, underlying disease cannot be controlled adequately, and long term invasive mechanical ventilation is required. If VAP occurs (sometimes recurrent) in such cases, not all VAPs are critical illness. The severity of VAP can be evaluated according to qSOFA or APACHE-II score.
Procedures for clinical management
Step 1
Confirm the clinical diagnosis of HAP/VAP based on symptoms, signs, and imaging findings, AND differentiate it primarily from other febrile diseases accompanied by pulmonary opacities, AND assess the severity of disease (complicated with sepsis or not), possible pathogens and risk factors for antibiotic resistance.
Step 2
Collect lower respiratory tract secretions and blood samples for testing of pathogenic microorganisms and infection-related biomarkers as soon as possible, AND initiate empiric antimicrobial treatment immediately. The treatment regimen, including appropriate antimicrobial agents, monotherapy or combination therapy, loading dose and maintenance dose, should be determined according to physicochemical properties and pharmacokinetic/pharmacodynamics (PK/PD) parameters of each antimicrobial agent.
Step 3
Re-evaluate results of laboratory tests and response to the initial antimicrobial therapy 48–72 hours later, and manage the patient specifically as follows: (I) switch to targeted therapy (de-escalation or step-down) when early favorable treatment response is observed clinically, and meaningful positive result is obtained from microbiological tests; (II) make an attempt to discontinue antimicrobial agents when clinical condition is stable without sepsis or without positive microbiological cultural result; (III) carefully evaluate the clinical implication of positive results of microbiological culture when the patient is not improved clinically in order to clarify whether the identified microorganism is pathogenic, or if there is mixed bacterial infection, any complications or infection in sites other than lung. The antimicrobial regimen should be adjusted accordingly based on relevant factors such as coverage of pathogens, antibiotic resistance profile, the consistency between in vivo efficacy and in vitro antimicrobial activity, and PK/PD parameters of antimicrobial agents; (IV) further clarify the diagnosis with additional etiological tests and identify non-infectious causes when the patient is not improved clinically and microbiological culture shows negative results.
Step 4
Monitor clinical presentations and systemic levels of infection-related biomarkers dynamically. Assess the response to the measures in step 3, and decide the duration of antimicrobial therapy and other subsequent management.
Treatment
HAP/VAP is usually managed by an integrated approach including antimicrobial therapy, respiratory support technique, multiple organ support therapy, and non-antimicrobial pharmacotherapy. Antimicrobial therapy is the fundamental treatment modality, including empiric antimicrobial therapy and pathogen-specific (targeted) treatment.
Empiric antimicrobial treatment
Principles of empiric antimicrobial therapy
(I) Timing of antimicrobial therapy: empiric antimicrobial therapy should be administered as soon as possible after the clinical diagnosis of HAP/VAP is established and etiological testing is submitted. Appropriate antimicrobial therapy, if delayed, is still associated with increased mortality and longer length of hospital stay (111,112). Therefore, HAP/VAP patients should be managed empirically with antimicrobial agents as soon as possible (IIIA). (II) Properly evaluate the risk factors for MDR pathogens: the risk factors for common antibiotic-resistant pathogens of HAP/VAP are listed in Table 6. Additionally, Table 7 lists the risk factors for several specific common MDR pathogens.

Full table

Full table
Selection of antimicrobial agents for initial empiric therapy
The strategy of initial empiric antimicrobial therapy for HAP/VAP is described in Figures 1 and 2. Appropriate antimicrobial agents should be selected in terms of disease severity, pathogen distribution in local hospital, antibiotic resistance profile, and risk factors for resistant pathogens, meanwhile taking the clinical characteristics, underlying diseases, and organ function of patients, PK/PD properties of antimicrobial agents, prior antibiotic use, and history of drug allergy into account (Tables 8 and 9) (126). The etiological spectrum and antibiotic resistance profile vary greatly with geographical region and hospital grade in China. Therefore, the recommended treatment in this guideline is fundamental only. The specific regimen should be provided according to the following conditions: (I) periodically prepare and release the distribution and antibiogram data of HAP/VAP pathogens in local hospital (127-129). The specific empiric treatment regimen should be based on the pathogens distribution of HAP/VAP and susceptibility testing results in local hospital (115,130,131) (IIIA). (II) For the patients with MRSA colonization in respiratory tract or treated in a healthcare unit with high prevalence of MRSA, it is recommended to cover MRSA empirically (IIIC). (III) For the HAP/VAP patients with risk factors for MDR P. aeruginosa or other MDR gram negative bacilli or at high risk of death, it is recommended to combine two different classes of antimicrobial agents. For the HAP/VAP patients not critically ill and without risk factors for MDR pathogens, antimicrobial monotherapy is appropriate for empiric treatment (IIIA). (IV) It is recommended to reserve polymyxin and tigecycline only for the patients with risk factors for XDR gram negative pathogens. (V) In the HAP/VAP patients complicated with sepsis, the loading dose and maintenance dose of antimicrobial agents should be adjusted according to the physicochemical properties, PK/PD profiles of antimicrobial agents and severity of organ dysfunction (especially kidneys and liver).
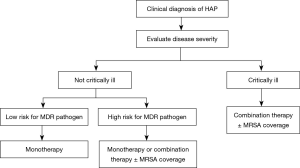
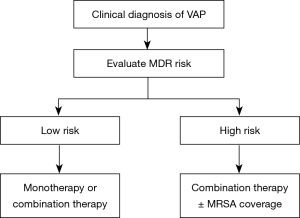
Full table

Full table
HAP/VAP pathogen-specific treatment
Pathogen-specific treatment is also known as targeted antimicrobial therapy, which means to prepare specific antimicrobial treatment regimen (narrow spectrum or broad spectrum, monotherapy or combination therapy) targeting the identified pathogenic microorganisms based on the results of in vitro antimicrobial susceptibility testing. Attention should be paid to the following points when prescribing HAP/VAP pathogen-specific antimicrobial therapy.
- Before initiation of an empirical antimicrobial treatment, qualified specimens should be submitted for microbiological testing, and evaluating the results of microbiological testing to rule out possible contamination or colonization.
- Adjust antimicrobial regimen appropriately according to the results of microbiological studies and susceptibility testing, as well as the efficacy of initial empiric therapy.
- XDR or PDR pathogens are usually found in HAP/VAP. In such cases, antimicrobial therapy should be given as early as possible, at adequate dosage, and in combination. The regimen, including specific dosage, mode and frequency of administration, should be prescribed based upon the minimum inhibitory concentration (MIC) value and PK/PD theory to optimize the effectiveness of antimicrobial treatment (132,133).
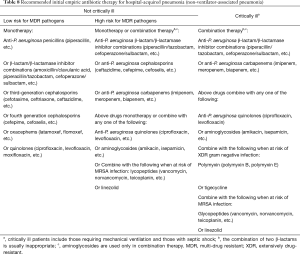
The recommended treatment regimen targeting the common drug-resistant pathogens of HAP/VAP is presented in Table 10.

Full table
Assessment of treatment efficacy and length of therapy
The length of antimicrobial therapy for HAP/VAP patients is generally 7 days or longer.
- Preliminary assessment of treatment efficacy: treatment response should be assessed 48–72 hours after initiation of empiric therapy. Treatment efficacy should be assessed comprehensively in terms of clinical symptoms and signs, imaging findings, and laboratory tests including infection-related markers. The treatment should be switched to pathogen-specific therapy or de-escalation (switching from combination therapy to monotherapy, or from broad spectrum to narrow spectrum antimicrobial agents) as soon as possible after learning the true pathogens (187-189) (IIIC). If empiric therapy fails and pathogen is unknown, additional microbiological tests are required to re-evaluate the probable pathogens, and adjust treatment regimen.
- Length of antimicrobial therapy: it should be determined according to multiple factors including severity of infection, specific pathogens and their resistance profiles, and clinical efficacy. The length of therapy is generally 7–8 days for the immunocompetent patients who receive appropriate initial empiric antimicrobial therapy with good clinical treatment response if the infection is caused by single pathogen without pulmonary emphysema, cystic fibrosis, cavity, necrotizing pneumonia, or lung abscess (8). For the patients failing to respond to initial antimicrobial therapy, and associated with critical illness, XDR or PDR pathogen, and lung abscess or necrotizing pneumonia, the length of therapy should be prolonged appropriately.
- Indications for stopping antimicrobial agents: discontinuation of antimicrobial therapy should be decided according to clinical symptoms and signs, imaging findings, and the results of laboratory tests (especially PCT) (IIIB).
Inhaled antimicrobial therapy
Inhaled antimicrobial therapy can be used in combination with systemic antimicrobial therapy when the following conditions are satisfied simultaneously: (I) HAP/VAP is caused by MDR GNB such as K. pneumoniae, P. aeruginosa, and A. baumannii; (II) systemic antimicrobial therapy alone cannot provide adequate therapeutic concentration in the site of infection, and thus have poor efficacy; (III) the inhaled antimicrobial agent to be used is active against the target pathogens (7,190,191) (IC). The inhaled antimicrobial agents are mainly aminoglycosides (including tobramycin and amikacin) and polymyxins (7,190-193).
Most of the clinical studies evaluating inhaled antimicrobial agents have major limitations, including small sample size, remarkable heterogeneity in study populations and dosing regimens. The clinical evidence of outcome improvement achieved by intravenous therapy in combination with inhaled antimicrobial agents is mainly seen in polymyxins. Therefore, the efficacy and safety of inhaled antimicrobial therapy require further evaluation (149,150,194,195).
No optimal regimen is available for inhaled antimicrobial agents currently. It is recommended to use polymyxin E at dose of 30–60 mg colistin base activity (CBA, equivalent to 1,000,000–2,000,000 IU), in 2–4 mL normal saline for IV administration, q8h-q12h (196,197). The recommended regimen of amikacin is 400 mg, bid or 25 mg/kg, once daily (198-200). The recommended regimen of tobramycin is 300 mg, q12h (172). The inhaled antimicrobial agents (especially polymyxin E) should be prepared immediately before use (197). The length of inhaled therapy is generally 14 d or until ventilator withdrawal. For the patients receiving mechanical ventilation, suitable atomization device/nebulizer should be selected, and appropriate oxygen concentration and preferred mode of ventilation is set properly according to the pathophysiological features of the patient (191,201).
Respiratory side effect of inhaled therapy is mainly airway spasm, which is characteristic of cough, wheezing, and dyspnea. The patient should be monitored for respiratory symptoms and oxygen saturation in the course of atomization. Atomization can be suspended in case of mild airway spasm, and bronchodilator should be given to alleviate the spasm. If airway spasm is persistent or severe, inhaled therapy should be stopped. The patients receiving nebulized aminoglycosides and polymyxin should be monitored for renal function, and therapeutic drug monitoring should be performed if possible. The patients under mechanical ventilation should be monitored for: (I) peak airway pressure, which will increase due to filter obstruction or airway spasm; (II) mental status. Low-dose sedatives can reduce patient-ventilator asynchrony, which should be discontinued timely at the end of atomization.
Adjuvant and supportive therapy
In addition to empiric and pathogen-specific antimicrobial therapies, it is also important for HAP/VAP patients to receive comprehensive management, including drainage of airway secretions, rational oxygen therapy, mechanical ventilation, fluid management, glycemic control, and nutritional support. Especially for critically ill patients, such adjuvant therapy usually can influence patient outcomes. Patients can benefit from reasonable use of adjuvant therapies.
Respiratory support techniques
(I) Drainage of airway secretions: airway secretions must be drained out timely and effectively so as to keep patency of the airway. This is the primary measures to support successful antimicrobial therapy of HAP/VAP, especially in critically ill patients complicated with lung abscess, empyema or poor airway clearance. Bedridden patients should be managed with regular body-turning and backslap, and active postural drainage in order to prevent aspiration. The patients are encouraged to take active respiratory function exercises (202). Extrasomatic vibration sputum discharge machine is appropriate for the patients with poor airway clearance and inadequate expectoration, to directly stimulate cough and sputum aspiration through nose/mouth or artificial airway, and sputum suction by bronchofibroscope if necessary. Bronchofibroscopic sputum suction should be implemented as early as possible for the patients receiving non-invasive mechanical ventilation with high output of sputum. This is helpful to reduce the rate of endotracheal intubation (203). (II) Rational oxygen therapy: oxygen therapy should be provided timely to the hypoxemic and severe HAP patients in order to keep arterial oxygen saturation (SaO2) >90%. Continuous oxygen therapy is required in the following cases: respiratory rate >24 breaths/min, PaO2 <60 mmHg, presence of shock or severe metabolic acidosis, and tissue hypoxia. High concentration oxygen therapy is appropriate for the patients with type I respiratory failure. Fraction of inspired oxygen (FiO2) ≥35% can increase PaO2 to above 60 mmHg or finger pulse oxygen saturation (SpO2) to above 90%. Low concentration continuous oxygen therapy (FiO2 <35%) should be administered routinely to the patients with type II respiratory failure to maintain PaO2 ≥60 mmHg or SpO2 ≥90%, and avoid significant increase of PaCO2. Other way of delivering oxygen therapy should be considered when PaCO2 increases significantly or PaO2 cannot be improved. There are many methods to deliver oxygen therapy, including traditional oxygen therapy (inhalation via nasal cannula or face mask), and high-flow nasal oxygen (HFNO). For critically ill HAP patients, HFNO may produce certain level of positive end-expiratory pressure (PEEP) because of inhalation of high oxygen flow, and associated with adequate humidification. It has gradually become an important way of oxygen therapy, and also as sequential treatment for patients after ventilator withdrawal and extubation, which has shown good efficacy and safety (204-206). (III) Mechanical ventilation: for the HAP patients with abnormal respiratory frequency (>30 breaths/min or <12 breaths/min), weak or absence of spontaneous breathing, seriously abnormal respiratory rhythm associated with disturbance of consciousness, use of accessory muscle with breathing or paradoxical breathing, mechanical ventilation should be considered timely when hypoxemia cannot be corrected even after HFNO therapy (207). Mechanical ventilation includes non-invasive and invasive mechanical ventilation. Non-invasive approaches assist ventilation primarily via oronasal mask or nasal mask, which are suitable for the conscious patients with stable vital signs and hemodynamics, and low volume of sputum, or the patients who can expectorate soberly. Pressure support ventilation (PSV) and bilevel positive airway pressure (BiPAP) are the frequently used non-invasive approaches of ventilation. The effect of ventilatory treatment can be evaluated in terms of the change of symptoms and signs, patient-ventilator synchrony, results of blood gas analysis, and other parameters. Appropriate use of non-invasive mechanical ventilation can reduce endotracheal intubation and the incidence of related complications, shorten the length of ICU stay (208). When the patient develops apparent abnormality of consciousness, poor drainage of sputum, abnormal hemodynamics, or respiratory failure indicated by blood gas analysis, the ventilation support should be switched to invasive mechanical ventilation in time. Invasive mechanical ventilation works mainly by endotracheal intubation (via mouth or nose) or tracheotomy, which is indicated for the HAP patients complicated with severe respiratory failure and/or abnormal vital signs, and meeting the following conditions: (i) Not indicated for non-invasive mechanical ventilation, and with severe life-threatening hypoxemia and/or hypercapnia (PaO2/FiO2 <150 mmHg); (ii) Inadequate clearance of airway secretions associated with high risk of aspiration (e.g., bulbar paralysis or abdominal distension, vomiting), disturbance of consciousness; (iii) hemodynamic instability, multiple organ failure; (iv) proper use of non-invasive mechanical ventilation fails to achieve the expected effect or even makes the condition worse. Non-invasive methods should not be used to replace invasive mechanical ventilation in the patients with clear indications for invasive mechanical ventilation, unless they refuse endotracheal intubation or tracheotomy. (IV) Extracorporeal membrane oxygenation (ECMO): if adequate conventional mechanical ventilation still cannot effectively improve the condition and reverse hypoxemia, ECMO should be considered as early as possible (209).
Multiple organ support therapy
(I) Hemodynamic monitoring and fluid management: critically ill HAP/VAP patients may experience insufficiency of effective circulating volume, and even septic shock at early stage due to fever, eating less, inflammatory response, and other factors. Their hemodynamic status should be evaluated dynamically as clinically indicated, and fluid resuscitation provided in time. Vasoactive drugs should be administered if necessary to maintain mean arterial pressure >65 mmHg. When large volume of crystalloid solution is required for fluid resuscitation, infusion of albumin can be considered as appropriate. (II) Glycemic control: blood glucose should be managed with reference to standard protocol. The target glucose level is ≤10 mmol/L. (III) Prevention of stress ulcer: in general, routine use of antacids is not recommended for prevention of stress ulcer. If patients have the risk factors for stress ulcer and gastrointestinal hemorrhage, they should be managed with gastric mucosal protective agents (e.g., sucralfate) and antacids, preferably proton pump inhibitors (PPIs), or H2 receptor antagonists (210). However, antacids may increase the incidence of HAP/VAP (211). (IV) Continuous renal replacement therapy (CRRT): currently, no consensus is reached in the timing, operating mode, parameter setting, and effect on patient outcomes for HAP/VAP patients to use CRRT. It is recommended to consider CRRT when HAP/VAP patients are complicated with septic shock and acute renal dysfunction. CRRT is helpful in eliminating metabolites in the body, managing liquid volume, correcting the disturbance of water, electrolytes, and acid-base balance, nutritional support, and clearance of some inflammatory mediators (212).
Non-antimicrobial pharmacotherapy
(I) Glucocorticoids: so far, no consensus is available for the timing, types, dosage, and duration of treatment regarding use of glucocorticoids in HAP/VAP patients. With reference to China CAP guideline (2016 Edition), it is recommended to use glucocorticoids only in critically ill HAP/VAP patients associated with hemodynamic instability. (II) Nutritional support: the HAP/VAP patients complicated with sepsis or septic shock should be supported with enteral nutrition as early as possible. If energy and protein intake don’t reach 60% of the target after enteral nutrition support for 7–10 days, parenteral nutrition supplements must be provided no matter what is the risk of malnutrition. If it is impossible to provide early (within 7 days since disease onset) enteral nutrition, and there is not risk of malnutrition, nutritional risk screening 2002 (NRS-2002) score ≤3, or nutrition risk in critically ill (NUTRIC) score ≤5, the patients should be supported by parenteral nutrition 7 days after disease onset. If there is risk of malnutrition or severe malnutrition, parenteral nutrition support should be initiated as early as possible (213,214). (III) Immunotherapy: there is controversy about the immunotherapy for HAP/VAP patients because we still lack clinical data from evidence-based medicine. In addition to antimicrobial therapy, critically ill HAP/VAP patients can be treated with immunoglobulin (0.5–1.0 g⸱kg−1⸱d−1) as appropriate, which may be helpful for controlling inflammatory reaction. Thymosin α1, an immunomodulator, may play a role in sepsis to improve immune paralysis (215).
Prevention
The overall strategy for HAP/VAP prevention is to minimize and control various risk factors. All healthcare activities must be consistent with the fundamental requirements and principles regarding disinfection, sterilization, and infection control in medical facilities. Medical staffs must be educated to improve their awareness of infection control, increase their compliance to hand hygiene, ensure careful disinfection and sterilization of medical devices and tools, strictly practise aseptic procedures, put target monitoring into place, and implement optimal use of antimicrobial agents.
Prevention of HAP
- Prevent aspiration: patients should be in semi-recumbent position (head-of-bed elevation 30–45°). The head of bed should not be too high to increase the risk of pressure sore. Feeding must be proper and reasonable.
- Reduce bacterial colonization in upper respiratory tract and/or gastrointestinal tract (216,217): oral care with chlorhexidine (Hibitane), chlorhexidine sponge bath, selective oropharyngeal decontamination (SOD), and use of probiotics.
- Actively treat underlying diseases: for critically ill patients, reinforce the nutritional support, promptly manage water, electrolytes, and acid-base imbalance, and control infection-related risk factors such as hypoproteinemia and hyperglycemia; active treatment and rehabilitation of cardiac and pulmonary diseases, adoption of airway clearance therapy (ACT) techniques, including breathing exercises, postural drainage, external manipulation or mechanical devices (218). More attention should be paid to the airway management of perioperative patients (especially those receiving thoracic and upper abdominal surgery). The respiratory tract must be kept humid and patent. Postoperative patients are encouraged to get out of bed as early as possible. Sedatives should be avoided if possible (219).
- Strengthen patient management: protective isolation should be provided to seriously immunocompromised patients such as organ transplant recipients and neutropenic patients. Contact precautions should be adopted for the patients infected or colonized with antibiotic-resistant microorganisms (e.g., MRSA, CRAB, CRPA, CRE) (143).
Prevention of VAP
There are specific risk factors and pathogenesis for VAP. Hence, in addition to the above-stated common preventive measures, the following specific precautions are necessary for prevention of VAP.
Prevent aspiration
The patients receiving invasive mechanical ventilation are recommended to raise the head of bed to 30–45° unless contraindicated (3,217,220) (IIA). Efforts should be made to help patients with sputum excretion by body-turning, machine vibration and percussion on back.
The subglottic cumulated secretions are the main source leading to aspiration in patients with artificial airway. The endotracheal tube with subglottic suction can reduce the incidence of VAP, and decrease the length of ICU stay (221-224). It is recommended to use such an endotracheal tube in the patients who are expected to undergo invasive ventilation for more than 48 or 72 hours (3,217) (IA). The cuff pressure should be kept at 25 cmH2O or higher (40,217,225) (IA). The subglottic secretions should be removed as clean as possible before balloon deflation or extubation.
Condensed fluid is usually formed in the pipelines of ventilator, which may facilitate bacterial growth and proliferation. Every effort should be made to avoid the condensate containing bacteria flowing directly into lower respiratory tract and so causing VAP, and also avoid its backflow into humidifier to allow the humidified bacteria-containing aerosol to be inhaled into lower respiratory tract. Condensate collecting bottle must be put at the lowest point of the pipelines, always kept upright, and made clean in time. Sterilized water should be used in the humidifier and nebulizer, and completely replaced every 24 hours. The circuit and accessories of ventilator should be patient-specific, and disinfected and sterilized every time after use. The patients receiving long-term mechanical ventilation are recommended to change ventilator circuit every week in general, but change immediately in case of visible filth or malfunction (217) (IIA).
The patients under mechanical ventilation should be supported by enteral nutrition as possible (3,217) (IIB). Enteral nutrition at early stage can promote intestinal movement, stimulate secretion of gastrointestinal hormones, improve intestinal blood perfusion, help maintain the structural integrity and barrier function of intestinal mucosa, and so reduce colonization and translocation of pathogens. It is better than parenteral nutrition. Post-pyloric feeding can reduce the incidence of VAP compared to gastric tube feeding, especially in the patients at high risk of aspiration. However, the mortality rate does not show significant difference between these two approaches of nutrition (226). Intermittent feeding and feeding with minimal gastric residual volume can reduce gastroesophageal reflux, decrease the risk and mortality of pneumonia. Gastrostomy can also reduce VAP incidence. Regular monitoring of gastric residual volume is not recommended for the patients who are asymptomatic and receiving enteral nutrition (217,227) (IIA).
Reduce colonization
Regular oral care is recommended for the patients who are receiving mechanical ventilation (217) (IIA), including oral rinse with gargles such as normal saline, chlorhexidine or povidone iodine solution, cleaning teeth and lingual surface with a toothbrush, q6h–q8h.
SOD means application of nonabsorbable antimicrobial agents to oropharynx, while selective digestive tract decontamination (SDD) indicates that nonabsorbable antimicrobial agents are used by application to oropharynx and oral administration, combined with parenteral antimicrobial agents or not. The aim of SOD and SDD is to eliminate the potential pathogens in oropharynx and gastrointestinal tract, which may cause secondary infection. Studies have shown that (228) either SOD (229) or SDD can reduce the incidence of HAP/VAP, and colonization of resistant pathoegns in respiratory tract. However, insufficient evidence is available to support its effect on decreasing the duration of mechanical ventilation, length of ICU stay, or reducing mortality. SDD may increase the risk of infection due to antibiotic-resistant bacteria, including Clostridium difficile infection. However, no study is available on the risk of long term use. SOD or SDD should be used cautiously in the patients under mechanical ventilation while considering the risk/benefit balance (3,217) (IIB).
Silver-coated endotracheal tube can reduce the incidence of VAP, but has no effect on the duration of mechanical ventilation, length of ICU stay, or mortality (230). At present, silver-coated endotracheal tube is not recommended for routine use (217) (IIB).
Oral administration of probiotics can reduce the incidence of VAP (231), but not mortality. Probiotics should be avoided in immunocompromised patients or the patients with gastrointestinal disorder at increased risk of bacterial translocation. In general, probiotics are not recommended for routine use to prevent VAP (3,217) (IIB).
Prevention of stress ulcer is one of the important measures to manage the patients under mechanical ventilation in ICU. The medicine commonly used in clinical practice include gastric mucosal protective agents (e.g., sucralfate) and antacids such as H2 receptor antagonists (H2RA), and PPIs. Gastric mucosal protective agents can reduce the risk of VAP compared to antacids, but only play a minor role in preventing gastrointestinal hemorrhage. It is currently considered that the use of antacids to prevent stress ulcer may increase bacterial colonization in gastrointestinal tract and airway, but do not have effect on the mortality of VAP. So antacids should be used as clinically indicated (232) (IIB).
Reduce the use of invasive ventilation
Establishment of artificial airway and use of mechanical ventilation are the most important risk factors for developing VAP, and endotracheal intubation makes the risk of pneumonia increase by 6–21 fold (233), especially repeated or prolonged intubation. Frequent replacing ventilator pipelines can further increase the risk of VAP (216,234,235). It is crucial to prevent VAP by minimizing the use of invasive ventilation, and reducing the duration of invasive ventilation (217) (IA).
The indications for endotracheal intubation or tracheotomy should be adhered to strictly. Non-invasive ventilation is preferred for the patients requiring ventilator-assisted ventilation. Non-invasive positive-pressure ventilation (NIPPV) should be used as early as possible in the patients with chronic obstructive pulmonary disease or congestive heart failure and complicated with hypercapnia or hypoxemia, which can reduce the use of endotracheal intubation (236,237), and thus reduce the incidence of VAP (IA). HFNO can be used in the patients with type I respiratory failure due to various causes (238,239), and some patients with mild type II respiratory failure to reduce the rate of endotracheal intubation and reintubation (240) (IA). It is important to note that the use of the respiratory support measures mentioned above should not delay the necessary endotracheal intubation and make the condition worse.
The use of sedatives should be reduced or avoided if possible during invasive ventilation. The patient should be evaluated on a daily basis to see if the sedatives in-use are necessary, and they should be discontinued as early as possible (IA). It is especially important to avoid the use of benzodiazepines (241). The patients should be aroused every day if indicated to administer spontaneous breathing trial, and evaluate whether the ventilator and endotracheal tube can be removed. The duration of mechanical ventilation should be minimized if possible in order to reduce the risk of VAP (242) (IA).
Bundle interventions
Current researches have shown that the following core interventions can reduce the mean duration of mechanical ventilation and days of hospital stay, incidence and mortality of VAP, and/or cost (217,243-252) (IA). The primary interventions are: (I) use non-invasive respiratory support techniques preferably if possible; (II) evaluate the necessity of invasive mechanical ventilation and endotracheal intubation every day, and remove ventilator or endotracheal tube as early as possible; (III) deep sedation should be avoided if possible in the patients who are receiving mechanical ventilation. If the use of sedatives is necessary, the patients should be aroused regularly to administer spontaneous breathing trial. The patients should be evaluated on a daily basis to see if the sedatives in-use are necessary, and they should be discontinued as early as possible; (IV) use endotracheal tube with subglottic suction in the patients who are expected to undergo mechanical ventilation for more than 48 or 72 hours; (V) Cuff pressure should be kept at 25 cmH2O or higher; (VI) the head of bed should be raised to 30–45° unless contraindicated; (VII) Strengthen oral care, preferably with chlorhexidine gargle; (VIII) ventilator circuit should be cleaned and disinfected appropriately. It is recommended to replace ventilator circuit every week in general, but replace immediately in case of visible filth or malfunction; (IX) strictly follow the aseptic techniques during airway-related procedures; (X) encourage and help the patients under mechanical ventilation to get out of bed and receive rehabilitation training as early as possible.
In addition to implementation of the above core interventions, the following precautions can be adopted selectively according to the characteristics of patient population and specific conditions in local ICU. Clinicians should collect evidence-based data and health economic information to support relevant preventive interventions, such as early tracheotomy for the patients under endotracheal intubation, prevention of stress ulcer, SOD/SDD, prophylactic use of probiotics, use of endotracheal tube made of special material (e.g., antibiotic-coated, silver-coated, or ultrathin polyurethane cuff). Closed endotracheal suction system has no effect on VAP incidence or other outcomes of patient (217), but it is useful for the control of respiratory infectious diseases which are disseminated via aerosol/air in hospitals.
Supplementary
Comments and recommendations regarding other issues related to HAP/VAP in this guideline
Healthcare-associated pneumonia (HCAP)
IDSA/ATS first proposed the concept of healthcare-associated pneumonia (HCAP) in 2005 (2), the aim of which is to timely identify infections caused by MDR pathogens from community-acquired pneumonia, and improve the outcome of this kind of pneumonia by empirically covering MDR microorganisms with broad spectrum antimicrobial agents. The clinical utility of this concept was supported by the data from large scale clinical studies in the US in the early days (253). However, as relevant studies are conducted extensively worldwide, controversies about the concept of HCAP are growing. European guidelines for the management of adult lower respiratory tract infections in 2011 state that the data currently available from evidence-based medicine don’t support the adoption of HCAP concept in Europe (254).
The controversy mainly focuses on the following two aspects: (I) there is great difference in the results of various HCAP etiological studies, and the results of most studies have shown that the concept of HCAP cannot exactly identify MDR infections (255). The prevalence of antibiotic-resistant pathogens in HCAP is generally lower in European and Asian countries than the level reported in the US (256-261). The meta-analysis recently published indicates that the sensitivity and specificity of using HCAP definition to screen antimicrobial-resistant infections don’t reach the threshold for its use in clinical practice (255). In fact, in addition to the risk factors mentioned in the definition of HCAP, there are still at least 10 additional risk factors which may increase the risk of antibiotic-resistant pneumonia. In specific study population, the combination of several of these risk factors usually performs better than HCAP in screening antibiotic-resistant infections (262). (II) The empiric, broad spectrum antimicrobial regimen recommended in 2005 IDSA/ATS guidelines cannot effectively improve the outcome of HCAP patients. Although the results of early studies suggested that the higher mortality of HCAP may be associated with inadequate coverage of antibiotic-resistant pathogens by initial treatment (263,264), most of the studies in recent years have indicated that the higher mortality rate of HCAP is primarily associated with advanced age, complications, severe underlying diseases, and organ dysfunction, but not necessarily with antibiotic-resistant pathogens. The broad spectrum antimicrobial treatment regimen recommended in 2005 IDSA/ATS guidelines could not shorten the length of hospital stay and stable duration of disease in HCAP patients (265). When the IDSA/ATS updated their HAP/VAP guidelines in 2016, all authors agreed unanimously that the concept of HCAP would no longer be accepted (7).
The structure of medical institutions in China is very different from that in the US. Even when the idea of HCAP is most popular, we did not follow the fashion to adopt this concept in China. The guidelines do not recommend the use of HCAP concept in China. The antibiotic resistance profile of local pathogens should be highly valued in the treatment of pneumonia patients. It is important to comprehensively analyze all the risk factors for antibiotic-resistant pathogens, rather than simply identify whether the pneumonia in a patient is HCAP or not (7) (IA).
Ventilator-associated tracheobronchitis (VAT)
It is generally considered that VAT is an intermediate step in the course of VAP pathogenesis when the microorganisms colonizing in lower respiratory tract become pathogens of VAP (266-269). At present, there is still controversy about whether VAT is an independent disease, and no uniform diagnostic criteria are available. It is still required to further clarify the exact boundary between microbial colonization, VAT, and VAP. Theoretically, in contrast to VAP, VAT is not associated with pulmonary parenchymal infiltration, and infrequently associated with decreased oxygenation capacity. Quantitative culture of the specimens from distal airway (PSB or BAL) also results in lower concentration of bacteria than that in VAP (267,268,270).
The pathogens and incidence of VAT, as well as the proportion of VAT progression to VAP vary with studies, medical institutions, and the different units in the same medical institution (266-268,270-275). The adverse effects of VAT include longer duration of mechanical ventilation (268,272-277), longer time of ICU stay (268,272-277) and total hospital stay (272,273), but the mortality is not affected significantly (266,271,273). Recently, several relevant studies and meta-analysis indicate that VAT is not an independent risk factor for increased mortality in the patients under mechanical ventilation (273). VAT also has no significant effect on the attributable mortality of the patients under mechanical ventilation (266). It is controversial about whether antimicrobial treatment is required for VAT (266,273,278). Studies have revealed that appropriate systemic antimicrobial treatment can reduce the proportion of VAT progression to VAP (266,270,273), but cannot reduce VAT mortality (266,273). Few clinical studies are available on the effect of inhaled antimicrobial agents in treatment of VAT. The quality of these studies is poor. The clinical significance of antimicrobial therapy on VAT still cannot be evaluated accurately based on the study data currently available (279,280).
The recommendations of 2016 IDSA/ATS HAP/VAP guidelines state that antimicrobial therapy is not required for VAT (7). This, to some extent, makes the diagnosis of VAT meaningless in clinical practice. Since no uniform and critically feasible diagnostic criteria are available for VAT, and the high quality evidence to support antimicrobial therapy for VAT is not enough yet, we also suggest not adopting VAT as a diagnosis in clinical practice and not providing antimicrobial therapy for VAT (IIB). Before the well-accepted diagnostic criteria and high quality evidence are available, considering VAT as an independent disease and prescribing antibiotics for patients with VAT may further increase the consumption of antimicrobial agents in ICU, which is unfavorable for curbing the spread of antimicrobial resistant pathogens, and may also increase the incidence of antibiotic-related adverse effects.
For the critically ill patients, among which chest CT scan cannot be performed and point-of-care chest X-ray film often cannot exclude pneumonia, if there are new respiratory signs and systemic signs suggesting VAP, empiric antimicrobial therapy may be administered prudently even in the absence of enough radiographic evidence to confirm VAP. It is not necessary to deliberately differentiate whether the patients are suffering VAT or VAP.
Early-onset and late-onset HAP/VAP
The results of early studies have shown that the airway-colonizing microorganisms in the hospitalized patients gradually transform from community-acquired pattern to hospital-acquired pattern 3–4 days after hospital admission (281). Corresponding to such a shift, it was considered that the pathogenic spectrum of HAP/VAP and their antibiotic resistance profile would vary with the onset time of pneumonia after admission (2). Traditionally, HAP/VAP is usually classified into early-onset (≤4 d) and late-onset HAP/VAP (≥5 d) in terms of the onset time since hospital admission (2). It was previously considered that as for early-onset HAP/VAP, if the patient doesn’t have other risk factors for MDR infection, the pathogen pattern is very similar to that isolated in CAP, while late-onset HAP/VAP are mostly caused by antibiotic-resistant pathogens, including P. aeruginosa, Acinetobacter spp., MDR Enterobacteriaceae or MRSA (2). In recent years, a series of clinical studies based on large sample size at home and abroad have reported that the composition of pathogens and prevalence of core pathogens are very similar between early-onset and late-onset HAP/VAP (18,282). It is not uncommon to isolate MDR pathogens in early-onset HAP/VAP (120,283,284).
In 2005 IDSA/ATS HAP/VAP guidelines, the onset time of pneumonia since hospital admission was set as one of the important criteria for patient stratification or formulation of empiric antimicrobial regimen (2). However, new evidence from clinical studies suggests that inappropriately emphasizing the effects of hospital stay length on HAP/VAP etiology may have unfavorable consequences on clinical outcomes. On the one hand, under-estimating the risk of antibiotic-resistant pathogens in the so-called early-onset HAP/VAP may lead to inadequate therapy, and so higher risk of failure of initial empiric antimicrobial therapy. On the other hand, for late-onset HAP/VAP, unnecessary overtreatment may be possible if only emphasizing the effects of onset time but neglecting detailed analysis of other true risk factors for antibiotic-resistant pathogens (250). Currently, it is widely accepted in China and abroad that prior intravenous antibiotic use 90 days before HAP/VAP onset is the most important risk factor for antibiotic-resistant pathogens, while the onset time since hospital admission only has relatively less effect on the risk of antibiotic-resistant infections.
In the Chinese “Guidelines for Diagnosis and Treatment of Hospital-acquired Pneumonia (draft)” released in 1999, the onset time was listed as one of the criteria for evaluating HAP with reference to the study results of foreign countries because the HAP/VAP survey data of our country were not available at that time (1). Subsequently, the results of our epidemiological survey didn’t show significant difference between the overall prevalence of various pathogens in early-onset HAP/VAP and that in late-onset HAP/VAP (18). For these reasons, this guideline recommends that empiric antimicrobial treatment should be based on the comprehensive analysis of risk factors for antibiotic-resistant pathogens, rather than the onset time of pneumonia alone (IIB).
HAP/VAP in immunocompromised host
HAP/VAP varies greatly between immunocompromised and immunocompetent subjects in spectrum of pathogens, clinical manifestations, and radiological signs. In addition to length of hospital stay, ICU stay, mechanical ventilation, antibiotic use, the prognosis of immunocompromised patients with HAP/VAP is also affected by type, severity, and duration of immunodeficiency (285-288).
Immunodeficiency can be classified into three types according to the impaired part of immune system, including neutropenia or neutrophils dysfunction, humoral immune defect, and cellular immune deficiency (286). Combined immunodeficiency may exist in some patients (286). In addition to the common HAP/VAP pathogens, opportunistic pathogens such as fungi (Aspergillus, Pneumocystis, Cryptococcus, Zygomycete, etc.), viruses (cytomegalovirus, herpes simplex virus, respiratory syncytial virus, etc.), Legionella and Nocardia are frequently found in HAP/VAP patients with severe neutropenia, or neutrophils dysfunction, serious cellular immune deficiency or combined immunodeficiency (285-291). HAP/VAP in immunocompromised patients is usually characterized by the following clinical features (285-288): insidious onset but rapid progression with poor outcomes and high mortality; earlier emergence and high incidence of dyspnea and respiratory failure; multiple lesions, sometimes diffuse lesion in the lungs; and high incidence of extrapulmonary breakthrough infections. In the patients with humoral immune defect alone, recurrent pyogenic infection caused by S. aureus, K. pneumoniae, Haemophilus influenzae, or P. aeruginosa are common. In the patients with cellular immunodeficiency, the pulmonary infections caused by M. tuberculosis, nontuberculous Mycobacteria, Nocardia, Aspergillus, or Cryptococcus are usually subacute or chronic disease, associated with single or multiple localized lesions in the lungs. Such infections are mostly community-acquired, and relatively fewer in HAP/VAP.
Clinical diagnosis of pneumonia is very challenging in immunocompromised patients due to the interference by underlying diseases and various treatment measures. It usually requires to be differentiated from some non-infectious pulmonary diseases (290,292). Chest CT scan can not only discover the occult lesions invisible on chest X ray film, but also reveal the imaging details of pulmonary lesions. CT scan can provide clues for estimating the possible pathogens, and guide the implementation of invasive procedures for etiological diagnosis (290,292-294). Therefore, when HAP/VAP is suspected in immunocompromised patients, it is recommended to perform chest CT scan instead of plain chest X film if possible (IIIC).
The etiology of pneumonia in immunocompromised patients is more diverse and complex than that in immunocompetent patients (285-291), so early and accurate etiological diagnosis is especially more important (285-288,290,292) (IIB). Routine smear, gram stain, and conventional bacterial culture of respiratory tract specimens are usually unable to allow accurate etiological diagnosis of pneumonia. So, additional etiological tests are required to identify fungi, viruses, Nocardia, Legionella, M. tuberculosis, and nontuberculous Mycobacteria, etc. according to the type of immunodeficiency and pulmonary imaging features (286,290,292). Bronchoalveolar lavage or TBLB (295), or percutaneous lung biopsy may be performed if necessary (296) to confirm diagnosis. Since the pathogens of infection secondary to immunodeficiency are required to be identified more quickly, and some opportunistic pathogens are difficult to be isolated, various special stains of respiratory tract specimens (smears), quick pathogen-specific antigen assay, and nucleic acid detection have more important diagnostic utility (286,292,297-302) (IIB). Detection of specific antibody in serum cannot allow early diagnosis, and false negative result is more frequent in immunocompromised patients, so its value is limited for immunocompromised patients (298,302) (IIIC).
The initial empiric antimicrobial therapy for HAP/VAP in immunocompromised patients should be based on the accurate assessment of the severity of pneumonia and immune deficiency. Efforts should be made to avoid both inadequate and delayed treatment due to under-estimation of the bad outcome of severe infection and/or serious immunodeficiency, and overtreatment due to indiscriminate “full coverage of pathogens”. When speculating the possible pathogens and selecting antimicrobial agents for initial empiric treatment, in addition to the factors conventionally considered in pneumonia patients such as local epidemiological data of HAP/VAP pathogens, the type, severity, and duration of immunodeficiency, as well as imaging features of pulmonary lesions should be also taken into account (285-288,290,292,299). Etiological tests should be promptly performed before initiation of antimicrobial therapy. Treatment efficacy should be evaluated timely. The initial treatment regimen should be adjusted appropriately according to the results of etiological tests.
In the treatment of the HAP/VAP secondary to immunodeficiency, immune reconstitution is an important measure and should be addressed seriously (286,290,292). The specific measures include facilitating recovery of neutrophil count, stopping or reducing the use of drugs which may result in immunosuppression, supplementing exogenous immune active agents, improving or controlling the diseases which may result in immunodeficiency (IIIC).
Acknowledgments
The following experts provided valuable opinions in the revision of this Guideline. Their efforts are also highly appreciated: Pharmacologists—Rui Wang (Chinese PLA General Hospital, Beijing, China), Yuan Lv (Peking University Third Hospital, Beijing, China); Experts of infectious diseases—Ming-Gui Wang (Huashan Hospital, Shanghai Medical College Fudan University, Shanghai, China), Bi-Jie Hu (Zhongshan Hospital, Shanghai Medical College Fudan University, Shanghai, China), Zheng-Yin Liu (Peking Union Medical College Hospital, Beijing, China), Fan Yang (Huashan Hospital, Shanghai Medical College Fudan University, Shanghai, China); Experts of critical care medicine—Er-Zhen Chen (Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Jian-Guo Li (Central South Hospital, Wuhan University, Wuhan, China), Hong-Ping Qu (Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Feng-Mei Guo (Nanjing Zhongda Hospital, Southeastern University School of Medicine, Nanjing, China), Ying-Zi Huang (Nanjing Zhongda Hospital, Southeastern University School of Medicine, Nanjing, China); Foreign experts—Mark L. Metersky, Co-first author, IDSA/ATS Guidelines for HAP/VAP (2016); Lionel A. Mandell, Co-chairman, IDSA/ATS Guidelines for CAP in Adults (2007); Shigeru Kono, Chairman, editorial board of Japanese Respiratory Society, 2017 Guideline for diagnosis and treatment of pneumonia in adults.
Funding: The study was supported by National Key R&D Program of China (granted number 2017YFC1309700, 2017 YFC13097001-4), Shanghai Key Discipline for Respiratory Diseases (granted number 2017ZZ02014) and innovative research team of high-level local universities in Shanghai.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Experts participating in panel discussion of this consensus document are (sorted by surnames alphabetically): Yu Chen (Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital of China Medical University, Shenyang, China), Yu-Sheng Chen (Department of Pulmonary and Critical Care Medicine, Fujian Provincial’s Hospital, Fuzhou, China), Hong-Bin Chen (Department of Clinical Laboratory Medicine, Peking University People’s Hospital, Beijing, China), Qi-Jian Cheng (Department of Pulmonary and Critical Care Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China), Xiang-Qun Fang (Department of Pulmonary and Critical Care Medicine, Chinese PLA General hospital, Beijing, China), Zhan-Cheng Gao (Department of Pulmonary and Critical Care Medicine, Peking University People’s Hospital, Beijing, China), Mei Jiang (State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease; Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangdong, China), Yu-Ping Li (Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, China), Er-Ran Li (Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of China Medical University, Shenyang, China), Hong Liu (Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China), Zhong-Sen Ma (Department of Pulmonary and Critical Care Medicine, The Second Affiliated Hospital of Jilin University, Jilin, China), Xiang-Dong Mou (Department of Pulmonary and Critical Care Medicine, Beijing Tsinghua Changgung Hospital, Medical Center, Tsinghua University, Beijing, China), Tie-Ying Sun (Department of Pulmonary and Critical Care Medicine, Beijing Hospital, National Center of Gerontology, Beijing, China), Jian Sun (Department of Pulmonary and Critical Care Medicine, The Fourth Affiliated Hospital of Nanchang University, Nanchang, China), Ning Shen (Department of Pulmonary Medicine, Peking University Third Hospital, Beijing, China), Chang-Zhou Shao (Department of Pulmonary and Critical Care Medicine, Zhongshan Hospital, Shanghai Medical College Fudan University, Shanghai, China), Zhao-Hui Tong (Department of Pulmonary and Critical Care Medicine, Beijing Institute of Respiratory Medicine and Beijing Chao-yang Hospital, Capital Medical University, China), Xin-Lun Tian (Department of Pulmonary and Critical Care Medicine, Peking Union Medical College Hospital, Beijing, China), Jing Wang (Department of Pulmonary and Critical Care Medicine, The first Affiliated Hospital of Zhengzhou University, Zhengzhou, China), Feng Xu (Department of Infectious Diseases, The Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China), Shu-Yun Xu (Department of Pulmonary and Critical Care Medicine, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China), Wei Zhang (Department of Pulmonary and Critical Care Medicine, The First Affiliated Hospital of Nanchang University, Nanchang, China), Lei Zhang (Department of Pulmonary and Critical Care Medicine, Dongfang Hospital, Xiamen University, Fuzhou, China), Qiao Zhang (Department of Pulmonary and Critical Care Medicine, Xinqiao hospital, The Third Military Medical University of PLA, Chongqing, China), Lan-Yan Zhu (Department of Pulmonary and Critical Care Medicine, The Second Xiangya Hospital of Central South University, Hunan, China), Yang-Qing Zhan(State Key Laboratory of Respiratory Disease, National Clinical Research Center for Respiratory Disease; Guangzhou Institute of Respiratory Health, the First Affiliated Hospital of Guangzhou Medical University, Guangzhou Medical University, Guangdong, China).
References
- Chinese Thoracic Society of Chinese Medical Association. Guideline for diagnosis and treatment of hospital-acquired pneumonia (draft). Chinese Journal of Tuberculosis and Respiratory Diseases 1999;14:160-1.
- American Thoracic S, Infectious Diseases Society of A. Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med 2005;171:388-416. [Crossref] [PubMed]
- Chinese Society of Critical Care Medicine, Chinese Medical Association. Guideline for diagnosis, prevention, and treatment of ventilator-associated pneumonia (2013). Chin J Intern Med 2013;52:524-43.
- Dalhoff K, Ewig S. Adult patients with nosocomial pneumonia: epidemiology, diagnosis, and treatment. Dtsch Arztebl Int 2013;110:634-40. [PubMed]
- Rotstein C, Evans G, Born A, et al. Clinical practice guidelines for hospital-acquired pneumonia and ventilator-associated pneumonia in adults. Can J Infect Dis Med Microbiol 2008;19:19-53. [Crossref] [PubMed]
- Masterton RG, Galloway A, French G, et al. Guidelines for the management of hospital-acquired pneumonia in the UK: Report of the Working Party on hospital-acquired pneumonia of the British Society for Antimicrobial Chemotherapy. J Antimicrob Chemother 2008;62:5-34. [Crossref] [PubMed]
- Kalil AC, Metersky ML, Klompas M, et al. Management of Adults With Hospital-acquired and Ventilator-associated Pneumonia: 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 2016;63:e61-e111. [Crossref] [PubMed]
- Torres A, Niederman MS, Chastre J, et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur Respir J 2017. [Crossref] [PubMed]
- Mikasa K, Aoki N, Aoki Y, et al. JAID/JSC Guidelines for the Treatment of Respiratory Infectious Diseases: The Japanese Association for Infectious Diseases/Japanese Society of Chemotherapy – The JAID/JSC Guide to Clinical Management of Infectious Disease/Guideline-preparing Committee Respiratory Infectious Disease WG. J Infect Chemother 2016;22:S1-65. [Crossref] [PubMed]
- Japanese Respiratory Society guidelines for management of pneumonia in adults (2017). Tokyo: Japanese Respiratory Society, 2017.
- Chinese Thoracic Society of Chinese Medical Association. Chinese guideline for diagnosis and treatment of community-acquired pneumonia in adults (2016 Edition). Chinese Journal of Tuberculosis and Respiratory Diseases 2016.253-79.
- Wu AH, Wen XM, Li CH, et al. China national point prevalence survey on healthcare-associated infection and antimicrobial use in 2012. Chinese Journal of Infection Control 2014;13:8-15.
- Ren N, Wen XM, Wu AH. Study on the changing trends in national nosocomial infection transection investigation results. Chinese Journal of Infection Control 2007;6:16-8.
- Magill SS, Edwards JR, Bamberg W, et al. Multistate Point- Prevalence Survey of Health Care-Associated Infections. New Engl J Med 2014;370:1198-208. [Crossref] [PubMed]
- Flanders SA, Collard HR, Saint S. Nosocomial pneumonia: state of the science. Am J Infect Control 2006;34:84-93. [Crossref] [PubMed]
- Micek ST, Chew B, Hampton N, et al. A Case-Control Study Assessing the Impact of Nonventilated Hospital-Acquired Pneumonia on Patient Outcomes. Chest 2016;150:1008-14. [Crossref] [PubMed]
- Burgmann H, Hiesmayr JM, Savey A, et al. Impact of nosocomial infections on clinical outcome and resource consumption in critically ill patients. Intensive Care Med 2010;36:1597-601. [Crossref] [PubMed]
- Liu YN, Cao B, Wang H, et al. Microbiological and clinical survey of hospital-acquired pneumonai in adults in 9 Chinese cities. Chinese Journal of Tuberculosis and Respiratory Diseases 2012;35:739-46. [PubMed]
- Rosenthal VD, Hu BJ, Maki DG, et al. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control 2012;40:396-407. [Crossref] [PubMed]
- Kollef MH, Hamilton CW, Ernst FR. Economic impact of ventilator-associated pneumonia in a large matched cohort. Infect Control Hosp Epidemiol 2012;33:250-6. [Crossref] [PubMed]
- Melsen WG, Rovers MM, Groenwold RH, et al. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis 2013;13:665-71. [Crossref] [PubMed]
- Gao XD, Hu BJ, Cui YW, et al. A multicenter prospective monitoring on incidences of ventilator-associated pneumonia in 46 hospitals in China. Chinese Journal of Infection Control 2015;14:540-3.
- Zhang DQ, Chen J, Liu JY, et al. Drug-resistance bacteria in patients with ventilator-associated pneumoniain intensive care unit between 2001 and 2010. Shanghai Medical Journal 2013:338-41.
- Ma J, Hu BJ, Gao XD, et al. Impact of bundle interventions on incidence of ventilator-associated pneumonia. Chinese Journal of Nosocomiology 2013;23:1540-2.
- Zhang JY, He QQ, Zhou B, et al. Risk factors for ventilator-associated pneumonia in ICU patients. Chinese Journal of Nosocomiology 2015.3467-9.
- Xie DS, Xiong W, Lai RP, et al. Ventilator-associated pneumonia in intensive care units in Hubei Province, China: a multicentre prospective cohort survey. J Hosp Infect 2011;78:284-8. [Crossref] [PubMed]
- Lin HC, Lin SM, Kuo CH, et al. Incidence and Outcome of Healthcare-Associated Acinetobacter baumannii in Chronically Ventilated Patients in a Tertiary Care Hospital in Taiwan. Am J Med Sci 2011;341:361-6. [Crossref] [PubMed]
- Chung DR, Song JH, Kim SH, et al. High Prevalence of Multidrug-Resistant Nonfermenters in Hospital-acquired Pneumonia in Asia. Am J Resp Crit Care Med 2011;184:1409-17. [Crossref] [PubMed]
- Kuti JL, Shore E, Palter M, et al. Tackling Empirical Antibiotic Therapy for Ventilator-Associated Pneumonia in Your ICU: Guidance for Implementing the Guidelines. Semin Respir Crit Care Med 2009;30:102-15. [Crossref] [PubMed]
- Blot S, Koulenti D, Dimopoulos G, et al. Prevalence, Risk Factors, and Mortality for Ventilator-Associated Pneumonia in Middle-Aged, Old, and Very Old Critically Ill Patients. Crit Care Med 2014;42:601-9. [Crossref] [PubMed]
- Makris D, Desrousseaux B, Zakynthinos E, et al. The impact of COPD on ICU mortality in patients with ventilator-associated pneumonia. Respir Med 2011;105:1022-9. [Crossref] [PubMed]
- Jaimes F, De La Rosa G, Gomez E, et al. Incidence and risk factors for ventilator-associated pneumonia in a developing country: where is the difference? Respir Med 2007;101:762-7. [Crossref] [PubMed]
- Muscedere JG, Day A, Heyland DK. Mortality, Attributable Mortality, and Clinical Events as End Points for Clinical Trials of Ventilator-Associated Pneumonia and Hospital-Acquired Pneumonia. Clin Infect Dis 2010;51:S120-5. [Crossref] [PubMed]
- Joseph NM, Sistla S, Dutta TK, et al. Ventilator-associated pneumonia: A review. Eur J Intern Med 2010;21:360-8. [Crossref] [PubMed]
- Bekaert M, Timsit JF, Vansteelandt S, et al. Attributable Mortality of Ventilator-Associated Pneumonia A Reappraisal Using Causal Analysis. Am J Respir Crit Care Med 2011;184:1133-9. [Crossref] [PubMed]
- Melsen WG, Rovers MM, Bonten MJM. Ventilator-associated pneumonia and mortality: A systematic review of observational studies. Crit Care Med 2009;37:2709-18. [PubMed]
- Zhang YM, Zheng YA, Guo ZG, et al. Clinical risk factors for ventilator-associated pneumonia in EICU. Chinese Journal of Nosocomiology 2015.3949-51.
- Chastre J, Fagon JY. Ventilator-associated pneumonia. Am J Respir Crit Care Med 2002;165:867-903. [Crossref] [PubMed]
- Herzig SJ, Howell MD, Ngo LH, et al. Acid-Suppressive Medication Use and the Risk for Hospital-Acquired Pneumonia. JAMA 2009;301:2120-8. [Crossref] [PubMed]
- Nseir S, Zerimech F, Fournier C, et al. Continuous Control of Tracheal Cuff Pressure and Microaspiration of Gastric Contents in Critically Ill Patients. Am J Resp Crit Care Med 2011;184:1041-7. [Crossref] [PubMed]
- Estes RJ, Meduri GU. The pathogenesis of ventilator-associated pneumonia: I. Mechanisms of bacterial transcolonization and airway inoculation. Intensive Care Med 1995;21:365-83. [Crossref] [PubMed]
- Quenot JP, Ladoire S, Devoucoux F, et al. Effect of a nurse-implemented sedation protocol on the incidence of ventilator-associated pneumonia. Crit Care Med 2007;35:2031-6. [Crossref] [PubMed]
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ 2016;353:i1585. [Crossref] [PubMed]
- Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med 2017;43:304-77. [Crossref] [PubMed]
- Chen HB, Zhao CJ, Wang H, et al. Distribution and drug resistance of pathogens causing hospital-acquired pneumonia from 2007 to 2013. Chinese Journal of Nosocomiology 2017;27:1-7.
- Zhang YZ, Liang YJ, Qin XG. Hospital acquired pneumonia in Grade II hospitals in Shanghai: a clinical analysis of 150 cases. Shanghai Medical Journal 2011;34:455-8.
- Liu J, Liu ZX, Wang W, et al. Distribution and drug resistance of pathogens causing hospital-acquired pneumonia in elderly patients. Chinese Journal of Nosocomiology 2013;23:2481-3.
- Xiao MS, Song NY. Distribution and drug resistance of pathogens causing hospital-acquired pneumonia in patients at advanced age. Chinese Journal of Gerontology 2010;30:3469-71.
- Chen GL, Wu HL, He YQ, et al. Distribution of ventilator-associated pneumonia pathogens in intensive care unit and drug resistance surveillance. Chinese Journal of Nosocomiology 2011;21:1244-6.
- He KY, Luo ZJ, Wang ZH, et al. Analysis of pathogenic and drug resistance of ventilator associated pneumonia in intensive care unit. Journal of Clinical Pulmonary Medicine 2017;22:138-41.
- Zhou ZW, Zou J. Analysis on the characteristics and drug resistance of ventilator-associated pneumonia pathogens. West China Medical Journal 2012:1471-3.
- Xue JL, Cai XY, Wang XR. Multicenter monitoring report on intensive care unit-acquired lower respiratory tract infection. Chinese Journal of Infection Control 2015;14:77-80.
- Xie YH. Risk factors and pathogens of ventilator-associated pneumonia in elderly patients. Journal of Clinical Medicine in Practice 2014;18:159-60.
- Zhang X, Yang YQ, Li YH, et al. Etiological and antibiotic resistance analysis of ventilator-associated pneumonia in elderly patients. Journal of Clinical Pulmonary Medicine 2013;18:1124-5.
- National Health and Family Planning Commission. Report on Antimicrobial Stewardship and Current Status of Antimicrobial Resistance in China (2017). Peking Union Medical College Publishing House 2017.
- Hu FP, Guo Y, Zhu DM, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005-2014. Clin Microbiol Infect 2016;22:S9. [Crossref] [PubMed]
- Tang X, Xiao M, Zhuo C, et al. Multi-level analysis of bacteria isolated from inpatients in respiratory departments in China. J Thorac Dis 2018;10:2666-75. [Crossref] [PubMed]
- Koenig SM, Truwit JD. Ventilator-Associated Pneumonia: Diagnosis, Treatment, and Prevention. Clin Microbiol Rev 2006;19:637. [Crossref] [PubMed]
- Wunderink RG, Woldenberg LS, Zeiss J, et al. The Radiologic Diagnosis of Autopsyproven Ventilator-associated Pneumonia. Chest 1992;101:458-63. [Crossref] [PubMed]
- Berlet T. Erratum to: Thoracic ultrasound for the diagnosis of pneumonia in adults: a meta-analysis. Respir Res 2015;16:104. [Crossref] [PubMed]
- Chavez MA, Shams N, Ellington LE, et al. Lung ultrasound for the diagnosis of pneumonia in adults: a systematic review and meta-analysis. Respir Res 2014;15:50. [Crossref] [PubMed]
- Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med 2012;38:577-91. [Crossref] [PubMed]
- Pauw BED, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Co. Betascript Publishing, 2008.
- Infection Study Group of Chinese Thoracic Society, Chinese Medical Association. Consensus Expert Group for diagnosis and treatment of Pneumomycosis. Chinese Journal of Tuberculosis and Respiratory Diseases 2007;30:821-34.
- Consensus Expert Group for differential diagnosis of febrile pulmonary opacity. Expert consensus on differential diagnosis of febrile pulmonary opacity. Chinese Journal of Tuberculosis and Respiratory Diseases 2016;39:169-76.
- Study Group of Pulmonary Vascular Diseases, Chinese Society of Cardiology, Chinese Medical Association. Chinese Expert Consensus on the Diagnosis and Treatment of Acute Pulmonary Thromboembolism. Chin J Intern Med 2010;49:74-81.
- Chinese Society of Critical Care Medicine. Chinese Medical Association. Guidelines for management of acute lung injury/acute respiratory distress syndrome: an evidence- based update by the Chinese Society of Critical Care Medicine (2006). Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2006;18:706-10. [PubMed]
- Chinese Society of Cardiology of Chinese Medical Association. Editorial Board of Chinese Journal of Cardiology. Chinese guidelines for diagnosis and treatment of heart failure in 2014. Zhonghua Xin Xue Guan Bing Za Zhi 2014;42:98-122. [PubMed]
- Berton DC, Kalil AC, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev 2007;10:CD006482.
- Group CCCT. A randomized trial of diagnostic techniques for ventilator-associated pneumonia. New Engl J Med 2006;355:2619-30. [Crossref] [PubMed]
- Brusselaers N, Labeau S, Vogelaers D, et al. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med 2013;39:365-75. [Crossref] [PubMed]
- Brun-Buisson C, Fartoukh M, Lechapt E, et al. Contribution of blinded, protected quantitative specimens to the diagnostic and therapeutic management of ventilator-associated pneumonia. Chest 2005;128:533-44. [Crossref] [PubMed]
- Raman K, Nailor MD, Nicolau DP, et al. Early antibiotic discontinuation in patients with clinically suspected ventilator-associated pneumonia and negative quantitative bronchoscopy cultures. Crit Care Med 2013;41:1656-63. [Crossref] [PubMed]
- Tong MQ. Standard operating procedures of blood culture in clinical microbiology laboratory. Chinese Journal of Laboratory Medicine 2004;27:124-6.
- Scholte JB, van Dessel HA, Linssen CF, et al. Endotracheal aspirate and bronchoalveolar lavage fluid analysis: interchangeable diagnostic modalities in suspected ventilator-associated pneumonia? J Clin Microbiol 2014;52:3597-604. [Crossref] [PubMed]
- Albert M, Friedrich JO, Adhikari NKJ, et al. Utility of Gram stain in the clinical management of suspected ventilator-associated pneumonia. Secondary analysis of a multicenter randomized trial. J Crit Care 2008;23:74-81. [Crossref] [PubMed]
- Gottesman T, Yossepowitch O, Lerner E, et al. The accuracy of Gram stain of respiratory specimens in excluding Staphylococcus aureus in ventilator-associated pneumonia. J Crit Care 2014;29:739-42. [Crossref] [PubMed]
- Hashimoto S, Shime N. Evaluation of semi-quantitative scoring of Gram staining or semi-quantitative culture for the diagnosis of ventilator-associated pneumonia: a retrospective comparison with quantitative culture. J Intensive Care 2013;2:1-5. [PubMed]
- O'Horo JC, Safdar N. Is the gram stain useful in the microbiologic diagnosis of VAP? A meta-analysis. Clin Infect Dis 2012;55:551-61. [Crossref] [PubMed]
- Seligman R, Seligman BG, Konkewicz L, et al. Accuracy of tracheal aspirate gram stain in predicting Staphylococcus aureus infection in ventilator-associated pneumonia. BMC Anesthesiol 2015;15:19. [Crossref] [PubMed]
- Liao XY, Ran Y, Bian SC, et al. Value of optimized point-of-care sputum smear in early management of ventilator-associated pneumonia. Chinese Critical Care Medicine 2014:879-83.
- Fagon JY, Chastre J, Wolff M, et al. Invasive and Noninvasive Strategies for Management of Suspected Ventilator-Associated Pneumonia: A Randomized Trial. Ann Intern Med 2000;132:621-30. [Crossref] [PubMed]
- Rea-Neto A, Youssef NC, Tuche F, et al. Diagnosis of ventilator-associated pneumonia: a systematic review of the literature. Crit Care 2008;12:R56. [Crossref] [PubMed]
- Torres A, Ewig S. Diagnosing ventilator-associated pneumonia. New Engl J Med 2004;350:433-5. [Crossref] [PubMed]
- Garrouste-Orgeas M, Timsit JF, Tafflet M, et al. Excess Risk of Death from Intensive Care Unit—Acquired Nosocomial Bloodstream Infections: A Reappraisal. Clin Infect Dis 2006;42:1118-26. [Crossref] [PubMed]
- Lim SJ, Choi JY, Lee SJ, et al. Intensive care unit-acquired blood stream infections: a 5-year retrospective analysis of a single tertiary care hospital in Korea. Infection 2014;42:875-81. [Crossref] [PubMed]
- Luna CM, Videla A, Mattera J, et al. Blood cultures have limited value in predicting severity of illness and as a diagnostic tool in ventilator-associated pneumonia. Chest 1999;116:1075. [Crossref] [PubMed]
- Mathur P, Varghese P, Tak V, et al. Epidemiology of Blood Stream Infections at a Level-1 Trauma Care Center of India. J Lab Physicians 2014;6:22-7. [Crossref] [PubMed]
- O'Keefe GE, Caldwell E, Cuschieri J, et al. Ventilator-associated pneumonia: bacteremia and death after traumatic injury. J Trauma Acute Care Surg 2012;72:713-9. [Crossref] [PubMed]
- Afshinnekoo E, Chou C, Alexander N, et al. Precision Metagenomics: Rapid Metagenomic Analyses for Infectious Disease Diagnostics and Public Health Surveillance. J Biomol Tech 2017;28:40-5. [Crossref] [PubMed]
- Grumaz S, Stevens P, Grumaz C, et al. Next-generation sequencing diagnostics of bacteremia in septic patients. Genome Med 2016;8:73. [Crossref] [PubMed]
- Long Y, Zhang Y, Gong Y, et al. Diagnosis of Sepsis with Cell-free DNA by Next-Generation Sequencing Technology in ICU Patients. Arch Med Res 2016;47:365. [Crossref] [PubMed]
- Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206-17. [Crossref] [PubMed]
- Infectious Disease Specialty Committee of China Medical Education Association. Expert consensus on interpretation of the clinical significance of infection-related biomarkers. Chinese Journal of Tuberculosis and Respiratory Diseases 2017;40:243-57.
- Falk G, Fahey T. C-reactive protein and community-acquired pneumonia in ambulatory care: systematic review of diagnostic accuracy studies. Fam Pract 2009;26:10-21. [Crossref] [PubMed]
- Lelubre C, Anselin S, Boudjeltia KZ, et al. Interpretation of C-Reactive Protein Concentrations in Critically Ill Patients. Biomed Res Int 2013;2013:124021. [Crossref] [PubMed]
- Sotillo-Díaz JC, Bermejo-López E, García-Olivares P, et al. Role of plasma procalcitonin in the diagnosis of ventilator-associated pneumonia: systematic review and metaanalysis. Medicina Intensiva 2014;38:337-46. [PubMed]
- Ye F, Zhong NS. Procalcitonin: a reliable marker fur guiding the management of severe bacterial infections. Chinese Journal of Tuberculosis and Respiratory Diseases 2012;35:873-6.
- Schuetz P, Amin DN, Greenwald JL. Role of procalcitonin in managing adult patients with respiratory tract infections. Chest 2012;141:1063. [Crossref] [PubMed]
- Liu D, Su LX, Guan W, et al. Prognostic value of procalcitonin in pneumonia: A systematic review and meta‐analysis. Respirology 2016;21:280. [Crossref] [PubMed]
- Pugh R, Grant C, Cooke RP, et al. Short-course versus prolonged-course antibiotic therapy for hospital-acquired pneumonia in critically ill adults. Cochrane Database Syst Rev 2015;8:CD007577. [PubMed]
- Schuetz P, Briel M, Christ-Crain M, et al. Procalcitonin to guide initiation and duration of antibiotic treatment in acute respiratory infections: an individual patient data meta-analysis. Clin Infect Dis 2012;55:651. [Crossref] [PubMed]
- Stolz D, Smyrnios N, Eggimann P, et al. Procalcitonin for reduced antibiotic exposure in ventilator-associated pneumonia: a randomised study. Eur Respir J 2009;34:1364. [Crossref] [PubMed]
- Zielińska-Borkowska U, Skirecki T, Złotorowicz M, et al. Procalcitonin in early onset ventilator-associated pneumonia. J Hosp Infect 2012;81:92. [Crossref] [PubMed]
- Larsson J, Itenov TS, Bestle MH. Risk prediction models for mortality in patients with ventilator-associated pneumonia: A systematic review and meta-analysis. J Crit Care 2017;37:112. [Crossref] [PubMed]
- Combes A, Luyt CE, Fagon JY, et al. Early predictors for infection recurrence and death in patients with ventilator-associated pneumonia. Crit Care Med 2007;35:146-54. [Crossref] [PubMed]
- Gursel G, Demirtas S. Value of APACHE II, SOFA and CPIS scores in predicting prognosis in patients with ventilator-associated pneumonia. Respiration 2006;73:503-8. [Crossref] [PubMed]
- Huang KT, Tseng CC, Fang WF, et al. An early predictor of the outcome of patients with ventilator-associated pneumonia. Chang Gung Med J 2010;33:274. [PubMed]
- Piskin N, Aydemir H, Oztoprak N, et al. Inadequate treatment of ventilator-associated and hospital-acquired pneumonia: risk factors and impact on outcomes. BMC Infect Dis 2012;12:268. [Crossref] [PubMed]
- Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016;315:762. [Crossref] [PubMed]
- Iregui M, Ward S, Sherman G, et al. Clinical Importance of Delays in the Initiation of Appropriate Antibiotic Treatment for Ventilator-Associated Pneumonia. Chest 2002;122:262-8. [Crossref] [PubMed]
- Luna CM, Aruj P, Niederman MS, et al. Appropriateness and delay to initiate therapy in ventilator-associated pneumonia. Eur Respir J 2006;27:158-64. [Crossref] [PubMed]
- Bouza E, Giannella M, Bunsow E, et al. Ventilator-associated pneumonia due to meticillin-resistant Staphylococcus aureus: risk factors and outcome in a large general hospital. J Hosp Infect 2012;80:150-5. [Crossref] [PubMed]
- Giunta V, Ferrer M, Esperatti M, et al. ICU-Acquired Pneumonia With or Without Etiologic Diagnosis: A Comparison of Outcomes. Crit Care Med 2013;41:2133-43. [Crossref] [PubMed]
- Montero M, Sala M, Riu M, et al. Risk factors for multidrug-resistant Pseudomonas aeruginosa acquisition. Impact of antibiotic use in a double case-control study. Eur J Clin Microbiol Infect Dis 2010;29:335-9. [Crossref] [PubMed]
- Parker CM, Kutsogiannis J, Muscedere J, et al. Ventilator-associated pneumonia caused by multidrug-resistant organisms or Pseudomonas aeruginosa: Prevalence, incidence, risk factors, and outcomes. J Crit Care 2008;23:18-26. [Crossref] [PubMed]
- Routsi C, Pratikaki M, Platsouka E, et al. Risk factors for carbapenem-resistant Gram-negative bacteremia in intensive care unit patients. Intensive Care Med 2013;39:1253-61. [Crossref] [PubMed]
- Swaminathan M, Sharma S, Blash SP, et al. Prevalence and Risk Factors for Acquisition of Carbapenem-Resistant Enterobacteriaceae in the Setting of Endemicity. Infect Control Hosp Epidemiol 2013;34:809-17. [Crossref] [PubMed]
- Wooten DA, Winston LG. Risk factors for methicillin-resistant Staphylococcus aureus in patients with community-onset and hospital-onset pneumonia. Respir Med 2013;107:1266-70. [Crossref] [PubMed]
- Martin-Loeches I, Deja M, Koulenti D, et al. Potentially resistant microorganisms in intubated paitents with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med 2013;39:672-81. [Crossref] [PubMed]
- Bassetti M, Peghin M, Pecori D. The management of multidrug-resistant Enterobacteriaceae. Curr Opin Infect Dis 2016;29:583-94. [Crossref] [PubMed]
- Einhorn AE, Neuhauser MM, Bearden DT, et al. Extended-spectrum beta-lactamases: Frequency, risk factors, and outcomes. Pharmacotherapy 2002;22:14-20. [Crossref] [PubMed]
- Malloy AMW, Campos JM. Extended-spectrum Beta-lactamases A Brief Clinical Update. Pediatr Infect Dis J 2011;30:1092-3. [Crossref] [PubMed]
- Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev 2005;18:657-86. [Crossref] [PubMed]
- Dangerfield B, Chung A, Webb B, et al. Predictive value of methicillin-resistant Staphylococcus aureus (MRSA) nasal swab PCR assay for MRSA pneumonia. Antimicrob Agents Chemother 2014;58:859-64. [Crossref] [PubMed]
- Sopena N, Sabria M, Grp NS. Multicenter study of hospital-acquired pneumonia in non-ICU patients. Chest 2005;127:213-9. [Crossref] [PubMed]
- Babcock HM, Zack JE, Garrison T, et al. Ventilator-associated pneumonia in a multi-hospital system: Differences in microbiology by location. Infect Control Hosp Epidemiol 2003;24:853-8. [Crossref] [PubMed]
- Beardsley JR, Williamson JC, Johnson JW, et al. Using local microbiologic data to develop institution-specific guidelines for the treatment of hospital-acquired pneumonia. Chest 2006;130:787-93. [Crossref] [PubMed]
- Rello J, Sa-Borges M, Correa H, et al. Variations in etiology of ventilator-associated pneumonia across four treatment sites - Implications for antimicrobial prescribing practices. Am J Respir Crit Care Med 1999;160:608-13. [Crossref] [PubMed]
- Dellit TH, Chan JD, Skerrett SJ, et al. Development of a guideline for the management of ventilator-associated pneumonia based on local microbiologic findings and impact of the guideline on antimicrobial use practices. Infect Control Hosp Epidemiol 2008;29:525-33. [Crossref] [PubMed]
- Fridkin SK, Edwards JR, Tenover FC, et al. Antimicrobial resistance prevalence rates in hospital antibiograms reflect prevalence rates among pathogens associated with hospital-acquired infections. Clin Infect Dis 2001;33:324-30. [Crossref] [PubMed]
- Lorente L, Jimenez A, Martin MM, et al. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 2009;33:464-8. [Crossref] [PubMed]
- Scaglione F, Esposito S, Leone S, et al. Feedback dose alteration significantly affects probability of pathogen eradication in nosocomial pneumonia. Eur Respir J 2009;34:394-400. [Crossref] [PubMed]
- Ye ZK, Chen YL, Chen K, et al. Therapeutic drug monitoring of vancomycin: a guideline of the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. J Antimicrob Chemother 2016;71:3020-5. [Crossref] [PubMed]
- Rybak MJ, Lomaestro BM, Rotschafer JC, et al. Vancomycin therapeutic guidelines: a summary of consensus recommendations from the infectious diseases Society of America, the American Society of Health-System Pharmacists, and the Society of Infectious Diseases Pharmacists. Clin Infect Dis 2009;49:325-7. [Crossref] [PubMed]
- Zhai SD, He B, Wang R, et al. Expert consensus on the therapeutic drug monitoring of vancomycin in China. Chinese Journal of Clinical Pharmacology 2016;32:1633-6.
- Mimoz O, Rolland D, Adoun M, et al. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med 2006;32:775-9. [Crossref] [PubMed]
- Wang JT, Liao HI, Wu LF, et al. Loading dose required to achieve rapid therapeutic teicoplanin trough plasma concentration in patients with multidrug-resistant gram-positive infections. Basic Clin Pharmacol Toxicol 2012;110:416-20. [Crossref] [PubMed]
- He LX, Pan J, Chen SY, et al. Clinical study of teicoplanin in the treatment of patients with gram-positive cocci: theChinese experience. Zhonghua Nei Ke Za Zhi 2005;44:337-41. [PubMed]
- Expert panel for consensus on teicoplanin dosage in clinical practice. Expert consensus on teicoplanin dosage in clinical practice. Chinese Journal of Tuberculosis and Respiratory Diseases 2016;39:500-8.
- Expert committee on management of vancomycin-resistant Enterococcus infections. Expert consensus on management of vancomycin-resistant Enterococcus infections. Chinese Journal of Experimental and Clinical Infectious Diseases 2010;4:60-4.
- Chen HZ, Lin GW, Wang JY. Practice of Internal Medicine. 14th Edition. People’s Medical Publishing House; 2013.
- Huang X, Deng ZD, Ni YX, et al. Chinese experts' consensus on prevention and control of multidrug resistance organism healthcare-associated infection. Chinese Journal of Infection Control 2015;14:1-9.
- Rubinstein E, Keynan Y. Vancomycin-resistant enterococci. Crit Care Clin 2013;29:841-52. [Crossref] [PubMed]
- Zhou H, Li GH, Chen BY, et al. Chinese Expert Consensus on the Strategy to Adress Extended Spectrum Beta-lactamases-Producing Entrobacteriaceae Infections. National Medical Journal of China 2014;94:1847-56.
- Rafailidis PI, Falagas ME. Options for treating carbapenem-resistant Enterobacteriaceae. Curr Opin Infect Dis 2014;27:479-83. [Crossref] [PubMed]
- Sharma R, Patel S, Abboud C, et al. Polymyxin B in combination with meropenem against carbapenemase-producing Klebsiella pneumoniae: pharmacodynamics and morphological changes. Int J Antimicrob Agents 2017;49:224-32. [Crossref] [PubMed]
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems?. Clin Microbiol Infect 2011;17:1135-41. [Crossref] [PubMed]
- Doshi NM, Cook CH, Mount KL, et al. Adjunctive aerosolized colistin for multi-drug resistant gram-negative pneumonia in the critically ill: a retrospective study. BMC Anesthesiol 2013;13:45. [Crossref] [PubMed]
- Tumbarello M, Pascale GD, Trecarichi EM, et al. Effect of aerosolized colistin as adjunctive treatment on the outcomes of microbiologically documented ventilator-associated pneumonia caused by colistin-only susceptible gram-negative bacteria. Chest 2013;144:1768-75. [Crossref] [PubMed]
- Falagas ME, Rafailidis PI, Matthaiou DK. Resistance to polymyxins: mechanisms, frequency and treatment options. Drug Resist Updat 2010;13:132. [Crossref] [PubMed]
- Morrill HJ, Pogue JM, Kaye KS, et al. Treatment Options for Carbapenem-Resistant Enterobacteriaceae Infections. Open Forum Infec Dis 2015;2:ofv050.
- Akova M, Daikos GL, Tzouvelekis L, et al. Interventional strategies and current clinical experience with carbapenemase-producing Gram-negative bacteria. Clin Microbiol Infect 2012;18:439-48. [Crossref] [PubMed]
- Falagas ME, Lourida P, Poulikakos P, et al. Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 2014;58:654-63. [Crossref] [PubMed]
- Lee GC, Burgess DS. Treatment ofKlebsiella PneumoniaeCarbapenemase (KPC) infections: a review of published case series and case reports. Ann Clin Microbiol Antimicrob 2012;11:32. [Crossref] [PubMed]
- Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015;70:2133. [Crossref] [PubMed]
- Tzouvelekis LS, Markogiannakis A, Piperaki E, et al. Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014;20:862-72. [Crossref] [PubMed]
- Zhanel GG, Lawson CD, Adam H, et al. Ceftazidime-Avibactam: a Novel Cephalosporin/β-lactamase Inhibitor Combination. Drugs 2013;73:159-77. [Crossref] [PubMed]
- Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Ch 2017:AAC.00883-17.
- MacVane SH, Crandon JL, Nichols WW, et al. In Vivo Efficacy of Humanized Exposures of Ceftazidime-Avibactam in Comparison with Ceftazidime against Contemporary Enterobacteriaceae Isolates. Antimicrob Agents Chemother 2014;58:6913-9. [Crossref] [PubMed]
- Falagas ME, Rafailidis PI, Ioannidou E, et al. Colistin therapy for microbiologically documented multidrug-resistant Gram-negative bacterial infections: a retrospective cohort study of 258 patients. Int J Antimicrob Agents 2010;35:194-9. [Crossref] [PubMed]
- Dalfino L, Puntillo F, Mosca A, et al. High-Dose, Extended-Interval Colistin Administration in Critically Ill Patients: Is This the Right Dosing Strategy? A Preliminary Study. Clin Infect Dis 2012;54:1720. [Crossref] [PubMed]
- Falagas ME, Tansarli GS, Ikawa K, et al. Clinical outcomes with extended or continuous versus short-term intravenous infusion of carbapenems and piperacillin/tazobactam: a systematic review and meta-analysis. Clin Infect Dis 2013;56:272. [Crossref] [PubMed]
- Levy Hara G, Gould I, Endimiani A, et al. Detection, treatment, and prevention of carbapenemase-producing Enterobacteriaceae: recommendations from an International Working Group. J Chemother 2013;25:129-40. [Crossref] [PubMed]
- Committee of Experts on Rational Drug Use, National Health and Family Planning Commission. Manual for management of antibiotic-resistant gram-negative bacterial infections. People’s Medical Publishing House; 2015.
- Ceccarelli G, Falcone M, Giordano A, et al. Successful Ertapenem-Doripenem Combination Treatment of Bacteremic Ventilator-Associated Pneumonia Due to Colistin-Resistant KPC-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2013;57:2900. [Crossref] [PubMed]
- Paul M, Carmeli Y, Durante-Mangoni E, et al. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 2014;69:2305-9. [Crossref] [PubMed]
- Wiskirchen DE, Crandon JL, Nicolau DP. Impact of various conditions on the efficacy of dual carbapenem therapy against KPC-producing Klebsiella pneumoniae. Int J Antimicrob Agents 2013;41:582-5. [Crossref] [PubMed]
- Fredborg M, Sondergaard TE, Wang M. Synergistic activities of meropenem double and triple combinations against carbapenemase-producing Enterobacteriaceae. Diagn Micr Infec Dis, 2017.
- Bulik CC, Nicolau DP. Double-Carbapenem Therapy for Carbapenemase-Producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011;55:3002. [Crossref] [PubMed]
- Giamarellou H, Galani L, Baziaka F, et al. Effectiveness of a double-carbapenem regimen for infections in humans due to carbapenemase-producing pandrug-resistant Klebsiella pneumoniae. Antimicrob Agents Chemother 2013;57:2388-90. [Crossref] [PubMed]
- Hallal A, Cohn SM, Namias N, et al. Aerosolized tobramycin in the treatment of ventilator-associated pneumonia: a pilot study. Surg Infect (Larchmt) 2007;8:73-82. [Crossref] [PubMed]
- Arnold HM, Sawyer AM, Kollef MH. Use of adjunctive aerosolized antimicrobial therapy in the treatment of Pseudomonas aeruginosa and Acinetobacter baumannii ventilator-associated pneumonia. Respir Care 2012;57:1226-33. [Crossref] [PubMed]
- Michalopoulos A, Fotakis D, Virtzili S, et al. Aerosolized colistin as adjunctive treatment of ventilator-associated pneumonia due to multidrug-resistant Gram-negative bacteria: A prospective study. Respir Med 2008;102:407. [Crossref] [PubMed]
- Guan X, He L, Hu B, et al. Laboratory diagnosis, clinical management and infection control of the infections caused by extensively drug resistant gram-negative bacilli: A Chinese consensus statement. Clin Microbiol Infect 2016;22 Suppl 1:S15. [Crossref] [PubMed]
- Dupont H, Marciniak S, Zogheib E, et al. Use of aztreonam in association with cefepime for the treatment of nosocomial infections due to multidrug-resistant strains of Pseudomonas aeruginosa to β-lactams in ICU patients: A pilot study. Anaesth Crit Care Pain Med 2015;34:141-4. [Crossref] [PubMed]
- Lv Y, Yan Z, Wang DH, et al. Optimized regimen of prolonged piperacillin/tazobactam infusion compared with conventional regimen in the treatment of hospital-acquired pneumonia. Chinese Critical Care Medicine 2013;25:479-83.
- Study Group of Infectious Diseases, Chinese Thoracic Society of Chinese Medical Association. Expert consensus on management of lower respiratory tract infections caused by P. aeruginosa. Chinese Journal of Tuberculosis and Respiratory Diseases 2014;37:9-15.
- Munozprice LS, Weinstein RA. Acinetobacter infection. New Engl J Med 2008;358:2846-7.
- Betrosian AP, Frantzeskaki FA, Douzinas E. Efficacy and safety of high-dose ampicillin/sulbactam vs. colistin as monotherapy for the treatment of multidrug resistant Acinetobacter baumannii ventilator-associated pneumonia. Journal of Infection 2008;56:432-6. [Crossref] [PubMed]
- Fishbain J, Peleg AY. Treatment of Acinetobacter infections. Clin Infect Dis 2010;51:79. [Crossref] [PubMed]
- Garnacho-Montero J, Amaya-Villar R. Multiresistant Acinetobacter baumannii infections: epidemiology and management. Curr Opin Infect Dis 2010;23:332. [Crossref] [PubMed]
- Chen BY, He LX, Hu BJ, et al. Expert consensus on management, prevention and control of A. baumannii infections in China. China Medicine and Pharmacy 2012;92:3-8.
- Neonakis IK, Spandidos DA, Petinaki E. Confronting multidrug-resistant Acinetobacter baumannii: a review. Int J Antimicrob Agents 2011;37:102. [Crossref] [PubMed]
- Zhou H, Li GH, Zhuo C, et al. Expert consensus on management, prevention, and control of Stenotrophomonas maltophilia infections in China. National Medical Journal of China 2013;93:1203-13.
- Abbott IJ, Slavin MA, Turnidge JD, et al. Stenotrophomonas maltophilia: emerging disease patterns and challenges for treatment. Expert Rev Anti Infect Ther 2011;9:471-88. [Crossref] [PubMed]
- Leone M, Bechis C, Baumstarck K, et al. De-escalation versus continuation of empirical antimicrobial treatment in severe sepsis: a multicenter non-blinded randomized noninferiority trial. Intensive Care Med 2014;40:1399-408. [Crossref] [PubMed]
- Joung MK, Lee JA, Moon SY, et al. Impact of de-escalation therapy on clinical outcomes for intensive care unit-acquired pneumonia. Crit Care 2011;15:R79. [Crossref] [PubMed]
- Kim JW, Chung J, Choi SH, et al. Early use of imipenem/cilastatin and vancomycin followed by de-escalation versus conventional antimicrobials without de-escalation for patients with hospital-acquired pneumonia in a medical ICU: a randomized clinical trial. Crit Care 2012;16:R28. [Crossref] [PubMed]
- Solé-Lleonart C, Roberts JA, Chastre J, et al. Global survey on nebulization of antimierobial agents in mechanically ventilated patients: a call for international guidelines. Clin Microbiol Infect 2016;22:359-64. [Crossref] [PubMed]
- Chinese Thoracic Society of Chinese Medical Association. Expert panel for drafting the expert consensus on the application of nebulizer therapy in respiratory diseases. Expert consensus on the application of nebulizer therapy in respiratory diseases. National Medical Journal of China 2016;96:2696-708.
- Kollef MH, Ricard JD, Roux D, et al. A Randomized Trial of the Amikacin Fosfomycin Inhalation System for the Adjunctive Therapy of Gram-Negative Ventilator-Associated Pneumonia IASIS Trial. Chest 2017;151:1239-46. [Crossref] [PubMed]
- Bassetti M, Luyt CE, Nicolau DP, et al. Characteristics of an ideal nebulized antibiotic for the treatment of pneumonia in the intubated patient. Ann Intensive Care 2016;6:35. [Crossref] [PubMed]
- Liu D, Zhang J, Liu HX, et al. Intravenous combined with aerosolised polymyxin versus intravenous polymyxin alone in the treatment of pneumonia caused by multidrug-resistant pathogens: a systematic review and meta-analysis. Int J Antimicrob Agents 2015;46:603-9. [Crossref] [PubMed]
- Abdellatif S, Trifi A, Daly F, et al. Efficacy and toxicity of aerosolised colistin in ventilator-associated pneumonia: a prospective, randomised trial. Ann Intensive Care 2016;6:26. [Crossref] [PubMed]
- Lu Q, Luo RB, Bodin L, et al. Efficacy of High-dose Nebulized Colistin in Ventilator-associated Pneumonia Caused by Multidrug-resistant Pseudomonas aeruginosa and Acinetobacter baumannii. Anesthesiology 2012;117:1335-47. [Crossref] [PubMed]
- European Medicines Agency completes review of polymyxin-based medicines. 2014. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/10/WC500176334.pdf. Accessed on May 08, 2016.
- Liu C, Zhang YT, Peng ZY, et al. Aerosolized Amikacin as Adjunctive Therapy of Ventilator-associated Pneumonia Caused by Multidrug-resistant Gram-negative Bacteria: A Single-center Randomized Controlled Trial. Chin Med J (Engl) 2017;130:1196-201. [Crossref] [PubMed]
- Luyt CE, Clavel M, Guntupalli K, et al. Pharmacokinetics and lung delivery of PDDS-aerosolized amikacin (NKTR-061) in intubated and mechanically ventilated patients with nosocomial pneumonia. Critical Care 2009;13:R200. [Crossref] [PubMed]
- Niederman MS, Chastre J, Corkery K, et al. BAY41-6551 achieves bactericidal tracheal aspirate amikacin concentrations in mechanically ventilated patients with Gram-negative pneumonia. Intensive Care Med 2012;38:263-71. [Crossref] [PubMed]
- Study Group of Respirtaory Therapeutics, Chinese Thoracic Society of Chinese Medical Association. Expert consensus on nebulizer therapy during mechanical ventilation (draft). Chinese Journal of Tuberculosis and Respiratory Disease 2014;37:812-5.
- Fink JB. Positioning versus postural drainage. Respir Care 2002;47:769-77. [PubMed]
- Scala R, Naldi M, Maccari U. Early fiberoptic bronchoscopy during non-invasive ventilation in patients with decompensated chronic obstructive pulmonary disease due to community-acquired-pneumonia. Critical Care 2010;14:R80. [Crossref] [PubMed]
- Matthay MA. Saving lives with high-flow nasal oxygen. N Engl J Med 2015;372:2225-6. [Crossref] [PubMed]
- Maggiore SM, Idone FA, Vaschetto R, et al. Nasal high-flow versus Venturi mask oxygen therapy after extubation. Effects on oxygenation, comfort, and clinical outcome. Am J Respir Crit Care Med 2014;190:282-8. [Crossref] [PubMed]
- Stéphan F, Barrucand B, Petit P, et al. High-Flow Nasal Oxygen vs Noninvasive Positive Airway Pressure in Hypoxemic Patients After Cardiothoracic Surgery: A Randomized Clinical Trial. JAMA 2015;313:2331-9. [Crossref] [PubMed]
- Mehta RM, Niederman MS. Nosocomial pneumonia in the intensive care unit: controversies and dilemmas. J Intensive Care Med 2003;18:175-88. [Crossref] [PubMed]
- Antonelli M, Conti G, Rocco M, et al. A comparison of noninvasive positive-pressure ventilation and conventional mechanical ventilation in patients with acute respiratory failure. N Engl J Med 1998;339:429-35. [Crossref] [PubMed]
- Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. N Engl J Med 2011;365:1905-14. [Crossref] [PubMed]
- Marik PE, Vasu T, Hirani A, et al. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010;38:2222-8. [Crossref] [PubMed]
- Lin PC, Chang CH, Hsu PI, et al. The efficacy and safety of proton pump inhibitors vs histamine-2 receptor antagonists for stress ulcer bleeding prophylaxis among critical care patients: a meta-analysis. Crit Care Med 2010;38:1197-205. [Crossref] [PubMed]
- Expert Panel for Consensus on Clinical Application of Blood Purification in Emergency Medicine. Expert consensus on clinical application of blood purification in emergency medicine. Chinese Journal of Emergency Medicine 2017;26:24-36.
- Heyland DK, Dhaliwal R, Drover JW, et al. Canadian clinical practice guidelines for nutrition support in mechanically ventilated, critically ill adult patients. JPEN J Parenter Enteral Nutr 2003;27:355-73. [Crossref] [PubMed]
- Society of Critical Care Medicine, Chinese Medical Association. Chinese guideline for management of severe sepsis/septic shock (2014). Clinical Education of General Practice 2015;54:401-26.
- Wu J, Zhou L, Liu J, et al. The efficacy of thymosin alpha 1 for severe sepsis (ETASS): a multicenter, single-blind, randomized and controlled trial. Crit Care 2013;17:R8. [Crossref] [PubMed]
- Kollef MH. Prevention of hospital-associated pneumonia and ventilator-associated pneumonia. Crit Care Med 2004;32:1396-405. [Crossref] [PubMed]
- Klompas M, Branson R, Eichenwald EC, et al. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol 2014;35:915-36. [Crossref] [PubMed]
- Volsko TA. Airway clearance therapy: finding the evidence. Respir Care 2013;58:1669-78. [Crossref] [PubMed]
- Clark DE, Lowman JD, Griffin RL, et al. Effectiveness of an early mobilization protocol in a trauma and burns intensive care unit: a retrospective cohort study. Phys Ther 2013;93:186-96. [Crossref] [PubMed]
- Wang L, Li X, Yang Z, et al. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst Rev 2016.CD009946. [PubMed]
- Muscedere J, Rewa O, McKechnie K, et al. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med 2011;39:1985-91. [Crossref] [PubMed]
- Mao Z, Gao L, Wang G, et al. Subglottic secretion suction for preventing ventilator-associated pneumonia: an updated meta-analysis and trial sequential analysis. Crit Care 2016;20:353. [Crossref] [PubMed]
- Damas P, Frippiat F, Ancion A, et al. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med 2015;43:22-30. [Crossref] [PubMed]
- Guo FL, Bu HJ, Yan HX, et al. Research of subglottic secretion drainage in severe respiratory-dysfunction patients receiving tracheostomy to prevent hospital acquired pneumonia. Chinese Journal of Practical Internal Medicine 2008;28:371-3.
- Nseir S, Lorente L, Ferrer M, et al. Continuous control of tracheal cuff pressure for VAP prevention: a collaborative meta-analysis of individual participant data. Ann Intensive Care 2015;5:43. [Crossref] [PubMed]
- Alkhawaja S, Martin C, Butler RJ, et al. Post-pyloric versus gastric tube feeding for preventing pneumonia and improving nutritional outcomes in critically ill adults. Cochrane Database Syst Rev 2015.CD008875. [PubMed]
- Reignier J, Mercier E, Le GA, et al. Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 2013;309:249. [Crossref] [PubMed]
- Pileggi C, Bianco A, Flotta D, et al. Prevention of ventilator-associated pneumonia, mortality and all intensive care unit acquired infections by topically applied antimicrobial or antiseptic agents: a meta-analysis of randomized controlled trials in intensive care units. Critical Care 2011;15:R155. [Crossref] [PubMed]
- Bergmans DC, Bonten MJ, Gaillard CA, et al. Prevention of ventilator-associated pneumonia by oral decontamination: a prospective, randomized, double-blind, placebo-controlled study. Am J Respir Crit Care Med 2001;164:382-8. [Crossref] [PubMed]
- Tokmaji G, Vermeulen H, Muller MC, et al. Silver-coated endotracheal tubes for prevention of ventilator-associated pneumonia in critically ill patients. Cochrane Database Syst Rev 2015.CD009201. [PubMed]
- Bo L, Li J, Tao T, et al. Probiotics for preventing ventilator-associated pneumonia. Cochrane Database Syst Rev 2014.CD009066. [PubMed]
- He H, Hu SL, Chen QH, et al. Effect of sucralfate and antacids on preventing stress ulcer and incidence of ventilator-associated pneumonia in patients under mechanical ventilation: a meta-analysis. Zhonghua Nei Ke Za Zhi 2014;53:48-54. [PubMed]
- Tablan OC, Anderson LJ, Arden NH, et al. Guideline for prevention of nosocomial pneumonia. The Hospital Infection Control Practices Advisory Committee, Centers for Disease Control and Prevention. Am J Infect Control 1994;22:247-92. [PubMed]
- Collard HR, Saint S, Matthay MA. Prevention of ventilator-associated pneumonia: an evidence-based systematic review. Ann Intern Med 2003;138:494-501. [Crossref] [PubMed]
- Eom CS, Jeon CY, Lim JW, et al. Use of acid-suppressive drugs and risk of pneumonia: a systematic review and meta-analysis. Canadian Medical Association Journal 2011;183:310-9. [Crossref] [PubMed]
- Chandra D, Stamm JA, Taylor B, et al. Outcomes of noninvasive ventilation for acute exacerbations of chronic obstructive pulmonary disease in the United States, 1998-2008. Am J Respir Crit Care Med 2012;185:152-9. [Crossref] [PubMed]
- Burns KE, Meade MO, Premji A, et al. Noninvasive ventilation as a weaning strategy for mechanical ventilation in adults with respiratory failure: a Cochrane systematic review. CMAJ 2014;186:E112-22. [Crossref] [PubMed]
- Frat JP, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. New Engl J Med 2015;372:2185. [Crossref] [PubMed]
- Maitra S, Som A, Bhattacharjee S, et al. Comparison of high-flow nasal oxygen therapy with conventional oxygen therapy and noninvasive ventilation in adult patients with acute hypoxemic respiratory failure: A meta-analysis and systematic review. J Crit Care 2016;35:138-44. [Crossref] [PubMed]
- Hernández G, Vaquero C, González P, et al. Effect of Postextubation High-Flow Nasal Cannula vs Conventional Oxygen Therapy on Reintubation in Low-Risk Patients: A Randomized Clinical Trial. JAMA 2016;315:1354. [Crossref] [PubMed]
- Klompas M, Li L, Szumita P, et al. Associations between different sedatives and ventilator-associated events, length-of-stay, and mortality in mechanically ventilated patients. Chest 2015;149:1373-9. [Crossref] [PubMed]
- Klompas M, Anderson D, Trick W, et al. The preventability of ventilator-associated events. The CDC Prevention Epicenters Wake Up and Breathe Collaborative. Am J Respir Crit Care Med 2015;191:292-301. [Crossref] [PubMed]
- Eom JS, Lee MS, Chun HK, et al. The impact of a ventilator bundle on preventing ventilator-associated pneumonia: a multicenter study. Am J Infect Control 2014;42:34-7. [Crossref] [PubMed]
- Shitrit P, Meirson M, Mendelson G, et al. Intervention to Reduce Ventilator-Associated Pneumonia in Individuals on Long-Term Ventilation by Introducing a Customized Bundle. J Am Geriatr Soc 2015;63:2089-93. [Crossref] [PubMed]
- Klompas M, Li L, Kleinman K, et al. Associations Between Ventilator Bundle Components and Outcomes. JAMA Intern Med 2016;176:1277. [Crossref] [PubMed]
- Ding S, Kilickaya O, Senkal S, et al. Temporal trends of ventilator-associated pneumonia incidence and the effect of implementing health-care bundles in a suburban community. Chest 2013;144:1461-8. [Crossref] [PubMed]
- Lim KP, Kuo SW, Ko WJ, et al. Efficacy of ventilator-associated pneumonia care bundle for prevention of ventilator-associated pneumonia in the surgical intensive care units of a medical center. J Microbiol Immunol Infect 2015;48:316-21. [Crossref] [PubMed]
- Rello J, Afonso E, Lisboa T, et al. A care bundle approach for prevention of ventilator-associated pneumonia. Clin Microbiol Infect 2013;19:363-9. [Crossref] [PubMed]
- Speck K, Rawat N, Weiner NC, et al. A systematic approach for developing a ventilator-associated pneumonia prevention bundle. Am J Infect Control 2016;44:652-6. [Crossref] [PubMed]
- Nair GB, Niederman MS. Ventilator-associated pneumonia: present understanding and ongoing debates. Intensive Care Med 2015;41:34-48. [Crossref] [PubMed]
- Kollef MH. Ventilator-Associated Pneumonia Prevention We Still Have a Long Way to Go! Chest 2014;146:873-4. [Crossref] [PubMed]
- Regulation for prevention and control of healthcare-associated infection in intensive care unit WS/T509-2016. Chinese Journal of Infection Control 2017;16:191-4.
- Kollef MH, Shorr A, Tabak YP, et al. Epidemiology and outcomes of health-care-associated pneumonia: results from a large US database of culture-positive pneumonia. Chest 2005;128:3854-62. [Crossref] [PubMed]
- Woodhead M, Blasi F, Ewig S, et al. Guidelines for the management of adult lower respiratory tract infections--full version. Clin Microbiol Infect 2011;17 Suppl 6:E1-59. [Crossref] [PubMed]
- Chalmers JD, Rother C, Salih W, et al. Healthcare-associated pneumonia does not accurately identify potentially resistant pathogens: a systematic review and meta-analysis. Clin Infect Dis 2014;58:330-9. [Crossref] [PubMed]
- Carratalà J, Mykietiuk A, Fernandez-Sabe N, et al. Health care-associated pneumonia requiring hospital admission: epidemiology, antibiotic therapy, and clinical outcomes. Arch Intern Med 2007;167:1393-9. [Crossref] [PubMed]
- Chalmers JD, Taylor JK, Singanayagam A, et al. Epidemiology, antibiotic therapy, and clinical outcomes in health care-associated pneumonia: a UK cohort study. Clin Infect Dis 2011;53:107-13. [Crossref] [PubMed]
- Dobler CC, Waterer G. Healthcare-associated pneumonia: A US disease or relevant to the Asia Pacific, too? Respirology 2013;18:923-32. [Crossref] [PubMed]
- Polverino E, Dambrava P, Cilloniz C, et al. Nursing home-acquired pneumonia: a 10 year single-centre experience. Thorax 2010;65:354-9. [Crossref] [PubMed]
- Qi F, Zhang GX, She DY, et al. Healthcare-associated Pneumonia: Clinical Features and Retrospective Analysis Over 10 Years. Chin Med J (Engl) 2015;128:2707-13. [Crossref] [PubMed]
- Venditti M, Falcone M, Corrao S, et al. Outcomes of patients hospitalized with community-acquired, health care-associated, and hospital-acquired pneumonia. Ann Intern Med 2009;150:19-26. [Crossref] [PubMed]
- Webb BJ, Dascomb K, Stenehjem E, et al. Predicting risk of drug-resistant organisms in pneumonia: moving beyond the HCAP model. Respir Med 2015;109:1-10. [Crossref] [PubMed]
- Shorr AF, Zilberberg MD. Role for risk-scoring tools in identifying resistant pathogens in pneumonia: reassessing the value of healthcare-associated pneumonia as a concept. Curr Opin Pulm Med 2015;21:232-8. [Crossref] [PubMed]
- Zilberberg MD, Shorr AF, Micek ST, et al. Antimicrobial Therapy Escalation and Hospital Mortality Among Patients With Health-Care-Associated Pneumonia A Single-Center Experience. Chest 2008;134:963-8. [Crossref] [PubMed]
- Troitino AX, Porhomayon J, El-Solh AA. Guideline-Concordant Antimicrobial Therapy for Healthcare-Associated Pneumonia: A Systematic Review and Meta-analysis. Lung 2013;191:229-37. [Crossref] [PubMed]
- Agrafiotis M, Siempos II, Falagas ME. Frequency, prevention, outcome and treatment of ventilator-associated tracheobronchitis: systematic review and meta-analysis. Respir Med 2010;104:325-36. [Crossref] [PubMed]
- Craven DE, Hudcova J, Rashid J. Antibiotic therapy for ventilator-associated tracheobronchitis: a standard of care to reduce pneumonia, morbidity and costs? Curr Opin Pulm Med 2015;21:250-9. [Crossref] [PubMed]
- Nseir S, Di Pompeo C, Pronnier P, et al. Nosocomial tracheobronchitis in mechanically ventilated patients: incidence, aetiology and outcome. Eur Respir J 2002;20:1483-9. [Crossref] [PubMed]
- Nseir S, Povoa P, Salluh J, et al. Is there a continuum between ventilator-associated tracheobronchitis and ventilator-associated pneumonia? Intensive Care Med 2016;42:1190-2. [Crossref] [PubMed]
- Nseir S, Martin-Loeches I, Makris D, et al. Impact of appropriate antimicrobial treatment on transition from ventilator-associated tracheobronchitis to ventilator-associated pneumonia. Crit Care 2014;18:R129. [Crossref] [PubMed]
- Dallas J, Skrupky L, Abebe N, et al. Ventilator-Associated Tracheobronchitis in a Mixed Surgical and Medical ICU Population. Chest 2011;139:513-8. [Crossref] [PubMed]
- Karvouniaris M, Makris D, Manoulakas E, et al. Ventilator-Associated Tracheobronchitis Increases the Length of Intensive Care Unit Stay. Infect Control Hosp Epidemiol 2013;34:800-8. [Crossref] [PubMed]
- Martin-Loeches I, Povoa P, Rodriguez A, et al. Incidence and prognosis of ventilator-associated tracheobronchitis (TAVeM): a multicentre, prospective, observational study. Lancet Respir Med 2015;3:859-68. [Crossref] [PubMed]
- Simpson VS, Bailey A, Higgerson RA, et al. Ventilator-Associated Tracheobronchitis in a Mixed Medical/Surgical Pediatric ICU. Chest 2013;144:32-8. [Crossref] [PubMed]
- Craven DE, Lei YX, Ruthazer R, et al. Incidence and Outcomes of Ventilator- associated Tracheobronchitis and Pneumonia. Am J Med 2013;126:542-9. [Crossref] [PubMed]
- Nseir S, Di Pompeo C, Soubrier S, et al. Outcomes of ventilated COPD patients with nosocomial tracheobronchitis: A case-control study. Infection 2004;32:210-6. [Crossref] [PubMed]
- Nseir S, Di Pompeo C, Soubrier S, et al. Effect of ventilator-associated tracheobronchitis on outcome in patients without chronic respiratory failure: a case-control study. Crit Care 2005;9:R238-45. [Crossref] [PubMed]
- Nseir S, Favory R, Jozefowicz E, et al. Antimicrobial treatment for ventilator-associated tracheobronchitis: a randomized, controlled, multicenter study. Crit Care 2008;12:R62. [Crossref] [PubMed]
- Palmer LB, Smaldone GC, Chen JJ, et al. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med 2008;36:2008-13. [Crossref] [PubMed]
- Russell CJ, Shiroishi MS, Siantz E, et al. The use of inhaled antibiotic therapy in the treatment of ventilator-associated pneumonia and tracheobronchitis: a systematic review. BMC Pulm Med 2016;16:40. [Crossref] [PubMed]
- Ewig S, Torres A, El-Ebiary M, et al. Bacterial colonization patterns in mechanically ventilated patients with traumatic and medical head injury. Incidence, risk factors, and association with ventilator-associated pneumonia. Am J Respir Crit Care Med 1999;159:188-98. [Crossref] [PubMed]
- Gastmeier P, Sohr D, Geffers C, et al. Early- and late-onset pneumonia: is this still a useful classification? Antimicrob Agents Chemother 2009;53:2714-8. [Crossref] [PubMed]
- Ferrer M, Liapikou A, Valencia M, et al. Validation of the American Thoracic Society-Infectious Diseases Society of America guidelines for hospital-acquired pneumonia in the intensive care unit. Clin Infect Dis 2010;50:945-52. [Crossref] [PubMed]
- Restrepo MI, Peterson J, Fernandez JF, et al. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir Care 2013;58:1220-5. [Crossref] [PubMed]
- Qu JM. The bewilderments and remark of diagnosis and management of pulmonary infections in immunocompromised patients in China. Chinese Journal of Practical Internal Medicine 2009.685-6.
- Japanese Respiratory S. Pneumonia in immunocompromised patients. Respirology 2009;14 Suppl 2:S44-50. [Crossref]
- Letourneau AR, Issa NC, Baden LR. Pneumonia in the immunocompromised host. Curr Opin Pulm Med 2014;20:272-9. [Crossref] [PubMed]
- Naccache JM. Pneumonia in the immunocompromised patient. Rev Prat 2011;61:1095-101. [PubMed]
- Evans SE, Ost DE. Pneumonia in the neutropenic cancer patient. Curr Opin Pulm Med 2015;21:260-71. [Crossref] [PubMed]
- Shorr AF, Susla GM, O'Grady NP. Pulmonary infiltrates in the non-HIV-infected immunocompromised patient: etiologies, diagnostic strategies, and outcomes. Chest 2004;125:260-71. [Crossref] [PubMed]
- Xu T, Tong ZH. Etiological pattern of pneumonia in immunocompromised patients. International Journal of Respiration 2014:1218-21.
- Maschmeyer G, Donnelly JP. How to manage lung infiltrates in adults suffering from haematological malignancies outside allogeneic haematopoietic stem cell transplantation. Br J Haematol 2016;173:179-89. [Crossref] [PubMed]
- Beigelman-Aubry C, Godet C, Caumes E. Lung infections: the radiologist's perspective. Diagn Interv Imaging 2012;93:431-40. [Crossref] [PubMed]
- Godet C, Elsendoorn A, Roblot F. Benefit of CT scanning for assessing pulmonary disease in the immunodepressed patient. Diagn Interv Imaging 2012;93:425-30. [Crossref] [PubMed]
- Brownback KR, Thomas LA, Simpson SQ. Role of bronchoalveolar lavage in the diagnosis of pulmonary infiltrates in immunocompromised patients. Curr Opin Infect Dis 2014;27:322-8. [Crossref] [PubMed]
- Haas BM, Clayton JD, Elicker BM, et al. CT-Guided Percutaneous Lung Biopsies in Patients With Suspicion for Infection May Yield Clinically Useful Information. AJR Am J Roentgenol 2017;208:459-63. [Crossref] [PubMed]
- Alanio A, Hauser PM, Lagrou K, et al. ECIL guidelines for the diagnosis of Pneumocystis jirovecii pneumonia in patients with haematological malignancies and stem cell transplant recipients. J Antimicrob Chemother 2016;71:2386-96. [Crossref] [PubMed]
- Chemaly RF, Shah DP, Boeckh MJ. Management of respiratory viral infections in hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2014;59 Suppl 5:S344-51. [Crossref] [PubMed]
- Godbole G, Gant V. Respiratory tract infections in the immunocompromised. Curr Opin Pulm Med 2013;19:244-50. [Crossref] [PubMed]
- Ljungman P, de la Camara R, Cordonnier C, et al. Management of CMV, HHV-6, HHV-7 and Kaposi-sarcoma herpesvirus (HHV-8) infections in patients with hematological malignancies and after SCT. Bone Marrow Transplant 2008;42:227-40. [Crossref] [PubMed]
- Mu XD, Wang GF, Su L. A clinical comparative study of polymerase chain reaction assay for diagnosis of pneumocystis pneumonia in non-AIDS patients. Chin Med J (Engl) 2011;124:2683-6. [PubMed]
- Vakil E, Evans SE. Viral Pneumonia in Patients with Hematologic Malignancy or Hematopoietic Stem Cell Transplantation. Clin Chest Med 2017;38:97-111. [Crossref] [PubMed]


