Combined laparoscopic and thoracoscopic Ivor Lewis esophagectomy using the transorally inserted anvil—the experience of Fujian Medical University Union Hospital
Introduction
Surgical approaches for treating esophageal carcinoma include the transhiatal esophagectomy (THE) and the transthoracic esophagectomy (TTE) with the anastomosis in the thorax or the neck. Compared with THE, TTE can resect more extensive intrathoracic esophageal tumors. Meanwhile, the range of the resected mediastinal lymph nodes with TTE is wider than THE. Performing an intrathoracic esophagogastric anastomosis using MIE techniques can be technically challenging and time-consuming (1,2). Combined laparoscopic with thoracoscopic Ivor-Lewis esophagectomy needs video-assisted mini-thoracotomy and the use of Endo-GIA stapler over and over. Due to the use of OrVil system, the operation of the intrathoracic esophagus anastomosis has become easy, and the operation time has reduced.
Patients and surgical technique
Patients
In total, 49 patients mean age 59.8±7.2 years with esophageal cancer who underwent a minimally invasive esophagectomy and esophagogastric anastomosis were recruited in the study (39 males and 10 females). Patients were initially staged with an endoscopy and computed tomography (CT) of the chest/abdomen/pelvis with oral and intravenous contrast. If no metastatic disease was detected in the CT scan, an esophageal endoscopic ultrasound (EUS) was performed to stage the depth of invasion and the regional lymphatic involvement. The tumors were located in the middle thoracic segment in 12 cases, and in the lower thoracic segment in 37 cases (including eight cases of esophagogastric junction adenocarcinoma involving the lower esophagus). The lesion length was 3.5±2.3 cm. All patients were diagnosed by gastroscopy and biopsy before the operation. The preoperative examination showed that the tumor had no obvious invasion, no obvious regional lymphadenopathy, and no distant metastasis.
Surgical technique
Step 1. Laparoscopic gastric conduit formation and placement of jejunal feeding tube
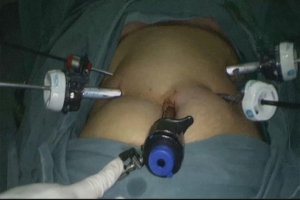
The laparoscopic operative position we take is the supine position without leg splitting. Five ports (1–1.5 cm) were made in the abdominal wall (Figure 1). First, we separated the gastrocolic ligament and short-gastric vessels and exposed the crus of the diaphragm adequately. The paracardiac lymph nodes were removed so the surgeon should avoid injury to right gastroepiploic vessels and the clone. We separated the attachments which connect the stomach with the pancreas and raised the stomach that the left gastric artery/vein so that it can be divided easily. Celiac lymph nodes were dissected out and sent separately. We resected the esophagus extendedly through the crevice into the lower mediastinum, laparoscopically. A gastric tube was formed with 4–5 cm of the proximal stomach excluded as a margin from the distal esophageal tumor. A 4–5 cm tube was made using a 60-mm endo straight cutting stapler in order to separate the lesser curvature preferably. All patients receive a jejunal feeding tube placement.
Step 2. Transthoracic dissection of esophagus, mediastinal lymphadenectomy, and esophagogastric anastomosis
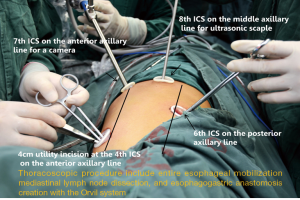
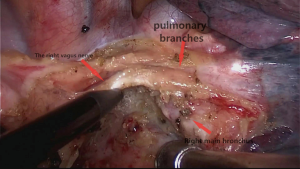
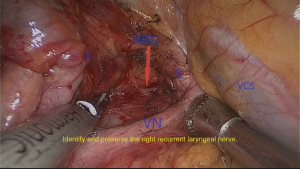
In order to make the adjacent ribs expand maximally, we keep the patients in the left lateral prone position with a break at the thorax. Meanwhile, we made four incisions from the axilla to the subscapular region which from T4 to T8 (Figure 2). Circumferential mobilization of the lower esophagus was undertaken with surrounding lymph nodes and perioesophageal tissue was moved to expose the pericardium anteriorly and aortic wall posteriorly. The azygos vein is separated. Meanwhile, the esophagus, lymph nodes, and periesophageal fat were separated circumferentially from the diaphragm to superior mediastinum, including the subcarinal and bilateral bronchial lymph nodes and the left and right para-recurrent laryngeal nerve lymph node. The pulmonary branches of the vagus nerve were protected while the esophageal branches were dissected (Figure 3). In the process of resecting the upper mediastinal lymph nodes, we used the method of mesoesophagus suspension which is more efficient. We resected the lymph nodes around the right recurrent laryngeal nerve with mesoesophagus suspension technique (Figure 4). Then we resected the lymph nodes around the right recurrent laryngeal nerve with mesoesophagus suspension technique while the structure of the upper mediastinal mesoesophagus is reserved (Figure 5).
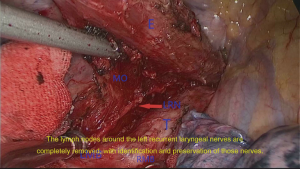
We use a 60-mm endo straight cutting stapler to cut the esophagus. The specimen is taken out in a bag. Once we confirmed the margins is negative by quick freezing pathology, we can perform the esophagogastric anastomosis. The OrVil EEA anvil, connected with an orogastric tube, is passed by the assistant transorally until the pressure at the staple line of the esophageal stump from the orogastric tube is visualized (3,4). An enterotomy should be performed in the middle of the esophageal stump when the pressure can be observed. It is quite critical to make the suitable enterotomy to allow suitable passage of the orogastric tube. The orogastric tube is separated from the anvil. We pull the tube out of the thorax carefully through a plastic trocar to prevent pollution (4).
We use the harmonic scalpel to make a gastrotomy at the superior aspect of the stapled gastric tube margin. We pass the 25-mm EEA circle stapler through the largest trocar site into the thoracic cavity (5). It is quite hard for patients whose rib spaces are narrow and small during this procedure. We put the stapler head into the conduit through the gastrotomy at the top of the gastric conduit. The EEA is fired, and the tissue rings can be evaluated for completeness. A 60-mm endo straight stapler is used to close the stump of the gastric conduit (Figure 6).

Postoperative care
We inserted a nasogastric tube into the gastric conduit and keep it in place close to the anastomosis until the anastomotic integrity was confirmed by a radiographic swallow evaluation, typically on post-operative day 7 (7). Two chest tubes were placed through two of the port sites. One of the two chest tubes was placed close to the anastomosis. We sutured the four thoracic ports and covered them with dressings. Patients can begin standard jejunal tube feeding within 24 hours. Patients should start with having a liquid diet until 14 to 21 days after the operation.
Comments
The procedure of the intrathoracic anastomosis should be the key step of a transthoracic MIE. The technique is particularly suited to the minimally invasive Ivor-Lewis esophagectomy. The OrVil is suited to patients who underwent the resectable middle or lower esophageal cancer without obvious swollen upper mediastinal and cervical lymph nodes found by perioperative CT scan. The mesentery of the esophagus contains the vessels, nerves, and regional lymph nodes (8). We adopt the method of mesoesophagus suspension to resect upper mediastinal lymph nodes adequately. Meanwhile, we do not dissect the upper mediastinal mesoesophagus. In this way, we can preserve proximal esophageal vessel which can provide enough blood supply for the esophagogastric anastomosis. The stapled anastomosis with the transoral anvil allows for a safe and reproducible anastomosis. According to our retrospective study, no death occurred, and no conversion to open surgery was needed. The average time of operation was 288.0±26.9 minutes, average intra-operative blood loss was 158.0±49.1 mL, average extubation time was 6.04±2.0 days, meantime to resume oral intake was 12.3±2.5 days, average postoperative hospital stay was 14.1±3.2 days. One patient had anastomotic leak in imageology but had no clinical symptoms (2.04%). The median follow-up was 11.1±4.4 months (range, 3–24 months) in 49 patients.
There has been an adequate opportunity in our medical center for thoracic surgical trainees to receive ample training in this complicated technique in a relatively short period. The surgical outcomes in the introduction period confirmed that an experienced surgeon could master these basic skills safely after 20 cases of esophageal cancer while working with the assistance of a regular surgical team (9).
Completely MIE and esophagogastric anastomosis with the technique of OrVil to treat middle and lower thoracic esophageal carcinoma is safe, feasible, and we can get better short-term learning curve.
Acknowledgments
Thanks Gaojie Lin for proofreading. This work was supported by Program for Innovative Research Team in Science and Technology in Fujian Province University.
Footnote
Conflicts of Interest: This video was granted the Award of Best Demonstration in the 2017 AME-Medtronic Minimally Invasive Esophageal Surgery Video Contest.
Informed Consent: Written informed consent was obtained from all patients for publication of this manuscript and any accompanying images.
References
- Jeong O, Park YK. Intracorporeal circular stapling esophagojejunostomy using the transorally inserted anvil (OrVil) after laparoscopic total gastrectomy. Surg Endosc 2009;23:2624-30. [Crossref] [PubMed]
- LaFemina J, Viñuela EF, Schattner MA, et al. Esophagojejunal reconstruction after total gastrectomy for gastric cancer using a transorally inserted anvil delivery system. Ann Surg Oncol 2013;20:2975-83. [Crossref] [PubMed]
- Campos GM, Jablons D, Brown LM, et al. A safe and reproducible anastomotic technique for minimally invasive Ivor Lewis oesophagectomy: the circular-stapled anastomosis with the trans-oral anvil. Eur J Cardiothorac Surg 2010;37:1421-6. [Crossref] [PubMed]
- Jaroszewski DE, Williams DG, Fleischer DE, et al. An early experience using the technique of transoral OrVil EEA stapler for minimally invasive transthoracic esophagectomy. Ann Thorac Surg 2011;92:1862-9. [Crossref] [PubMed]
- Li H, Hu B, You B, et al. Combined laparoscopic and thoracoscopic Ivor Lewis esophagectomy for esophageal cancer: initial experience from China. Chin Med J (Engl) 2012;125:1376-80. [PubMed]
- Zhang Z, Lin J, Zhuang L, et al. Ivor Lewis esophagectomy using Orvil. Asvide 2019;6:188. Available online: http://www.asvide.com/article/view/32486
- Kang M, Huang S, Lin J, et al. Video-assisted thoracoscopy the total mesoesophageal excision and systematic en bloc mediastinal lymph node dissection. J Vis Surg 2016;2:102. [Crossref] [PubMed]
- Tachimori Y. Total mesoesophageal esophagectomy. Chin Med J 2014;127:574-9. [PubMed]
- Lin J, Kang M, Chen C, et al. Thoracolaparoscopy oesophagectomy and extensive two-field lymphadenectomy for oesophageal cancer: introduction and teaching of a new technique in a high-volume centre. Eur J Cardiothorac Surg 2013;43:115-21. [Crossref] [PubMed]