Effects of different LAD-blocked sites on the development of acute myocardial infarction and malignant arrhythmia in a swine model
Introduction
Acute myocardial infarction (AMI) is a common cardiovascular disease characterized by rapid and sudden morbidity and high mortality (1). The occlusion positions of the coronary artery are closely correlated with the prognosis, among which the left anterior descending (LAD) is the most commonly blocked (2). LAD block can result in infarctions of the left anterior ventricular wall, cardiac apex and lateral wall. The prognosis varies because of different infarction areas in distinct LAD occlusion locations (3). AMI is often accompanied by malignant arrhythmia, such as ventricular tachycardia (VT) and ventricular fibrillation (VF), which account for 60-100% of deaths during the acute phase of AMI (4,5). Identification of the culprit vessel and blockage sites of AMI with malignant arrhythmia is one of the most heated research subjects.
Animal models provide opportunities for clinically study the pathophysiology and pathogenesis of AMI. The swine’s heart anatomy and coronary artery are close to those in humans (6,7), and great similarity exists between the swine AMI model established by blocking the coronary artery branch and AMI in humans (8). This present study employed the swine AMI model established by blocking the LAD through balloon angioplasty to explore the influence exerted by different LAD occlusion sites on the morbidity of AMI and malignant arrhythmia.
Materials and methods
Tested animals
Twenty-two male swine, weighing 26.32±2.15 kg and 3-4 months of age, were purchased from Jiangsu Academy of Agricultural Sciences and successfully passed inspection and quarantine.
Materials
Experimental equipment included an ECG monitor (V24E, Philips), a Labsystem electrical physiology recorder (Bard, USA), a VIVID 7 heart color ultrasonic diagnostic apparatus (GE, USA), a digital cardiovascular imaging system (Siemens, Germany), a cardiac reader analyzer (Roche, Switzerland), an i-STAT blood gas analyzer (Abbott, USA), a Vitros 5.1 FS automatic biochemical analyzer (Johnson & Johnson, USA), a Judkins Right 4.0 catheter, a guide wire for occlusion of the coronary artery with balloon angioplasty (Cordis, USA), a second catheter, a second guide wire for the punctuation of femoral artery, an artery sheath and a pressure pump (Medtronic).
Acute myocardial infarction model establishment
Twelve hours after a preoperative preparation, animals received oxygen inhalation (3 L/min), an intramuscular injection of ketamine (20-25 mg/kg) and atropine (0.5 mg) to induce anesthesia as described by our group previously (9,10). Swine were fixed in a supine position on the workstation, and access to the marginal ear vein was established. Pentobarbital sodium (3% solution; 60 mg/kg) was intravenously injected into the swine to induce a deep anesthetic state. During the operation, ketamine (100 mg) was injected intravenously every 20-30 min according to body motion to maintain anesthesia. After injection with 2% lidocaine for local anesthesia of the right groin of the swine, the right femoral artery was punctured to create AMI (11). Continuous ECG monitoring and vital sign changes were monitored during the procedure.
The animals were randomly divided into three groups according to the blocked sites of the balloon: group 1, control group (n=4), with catheter balloon inserted into the LAD, but without LAD occlusion; group 2, the bottom-third-blocked LAD group (n=9); and group 3, middle-site-blocked-LAD group (n=9). The femoral artery was punctured and a sheath was implanted with a guide wire inside. Diluted heparin (6,000 u) was injected intravenously and an additional 2,000 u was given every l h during the operation. The imaging tube was sent retrograde through the sheath to the coronary opening for angiography. The position for blocking was determined according to the imaging results for the bottom-third-blocked and middle-site-blocked-LAD groups. The balloon catheter (2.5-3.5 mm × 15 mm) with the guide wire was inserted through the sheath. The balloon was opened with six atmospheres to block the LAD for 90 minutes (12-14). Then the blood perfusion was restored. Continuous 12-lead ECG monitoring was applied during the whole procedure. If premature ventricular contractions (PVCs) were observed, the lidocaine and amiodarone dosage was increased. If VT was shown, intravenous lidocaine and amiodarone were given immediately. If VF appeared, an immediate 360 J external defibrillation and external chest compression was given until the restoration of sinus rhythm (15). Before and after the model creation, indexes including hemodynamics, ECG, myocardial enzymology and artery blood gas were collected to confirm the establishment of the model. The establishment of the model was assessed by ST elevation and the occurrence of pathologic Q wave, and confirmed by the pathological analyses of the hearts after the animals were sacrificed.
Arrhythmia monitoring and evaluation of AMI and ventricular fibrillation-cardiac arrest
Intra-operative continuous ECG monitoring was performed and the occurrence of ventricular arrhythmia was recorded. According to the international standard of arrhythmia (16), more than three continuous PVCs was defined as VT, less than 30 seconds VT was defined as non-sustained VT, and more than 30 seconds was defined as persistent VT; the disappearance of the equipotential line was defined as VF, which would lead to cardiac arrest if not treated promptly. Continuous VT and VF were defined as malignant arrhythmia.
Mean arterial blood pressure (MAP), left ventricular ejection fraction (LVEF), partial pressure of oxygen (PaO2) and carbon dioxide (PaCO2) in arteries were recorded before establishment of the model and 2 h after model establishment. Myoglobin (Mb), CK-MB, LDH, AST and cTnT were also collected 4 h after model establishment. Also, the QT and QTc intervals in each animal were measured and compared among three groups.
Statistical analyses
Statistical tests were performed in SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). Quantitative variables were expressed as mean ± SD and compared using an unpaired Student’s t-test or ANOVA test. Kruskal-Wallis H tests and Mann-Whitney U tests were used when the data did not met the normal distribution criteria or homogeneity of variance. Qualitative variables were compared using the χ2-test, unless otherwise indicated. A two-tailed P value of less than 0.05 was considered statistically significant.
Results
Model establishment of AMI and malignant arrhythmia
During the occlusion, the incidence of PVCs (88.89% vs. 100%, P=0.999) and VT (44.44% vs. 88.89%, P=0.134) were not significantly different between the bottom-third-blocked LAD group and the middle-site-blocked group, whereas the incidence of VF (33.33% and 100%, respectively, P=0.012) was significantly higher in the middle-site-blocked group. During the procedure, ECG showed that the ST segment was elevated and VT and VF occurred after LAD occlusion (Figure 1).
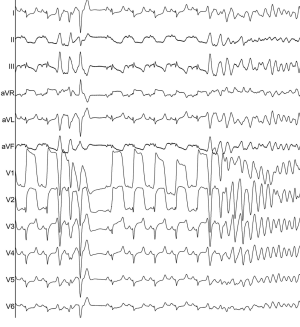
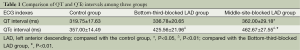
QTc interval comparisons
Compared with the Control group, QTc intervals in the Bottom-third-blocked LAD group and the middle-site-blocked group were significantly longer and the difference was statistically meaningful (bottom-third-blocked LAD group vs. control group, 425.56±21.96 vs. 357.00±14.49, P<0.01; middle-site-blocked group vs. control group, 462.67±27.55 vs. 357.00±14.49, P<0.01); QT intervals were statistically different between the middle-site-blocked group and the control group (362.00±29.18 vs. 319.75±17.63, P<0.05). The QTc interval was significantly increased in the middle-site-blocked LAD group when compared with that of the bottom-third-blocked LAD group (462.67±27.55 vs. 425.56±21.96, P<0.01), while QT intervals were not statistically different between these two groups (P>0.05) (Table 1).
Full table
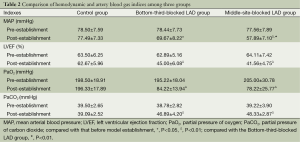
Comparison of hemodynamic and artery blood gas indexes before and after model establishment
Hemodynamic and artery blood gas indexes recorded after the model establishment suggested that MAP, LVEF and PaO2 indexes in the Bottom-third-blocked group and in the middle-site-blocked group were significantly lower than that before the model establishment, while PaCO2 was significantly higher. The differences in MAP, LVEF, PaO2 and PaCO2 indexes among the three groups before the model establishment were not statistically significant (P>0.05). However, after the occlusion, MAP in the middle-site-blocked group was significantly lower than that in the Bottom-third-blocked group (P<0.01), while LVEF, PaO2 and PaCO2 were not statistically different between the two groups (P>0.05) (Table 2).
Full table
Myocardial enzymology
Myocardial enzymology indexes recorded 4 h after the model establishment indicated that Mb, CK-MB, LDH, AST and cTnT in both the Bottom-third-blocked LAD group and the middle-site-blocked group were statistically higher than that in Control group (P<0.01) (Table 3). These indexes were not significantly different between the bottom-third-blocked LAD group and the middle-site-blocked group (P>0.05), which suggested that the difference in occlusion sections of LAD would not influence myocardial enzymology after AMI.

Full table
Discussion
Malignant arrhythmias are the main causes of sudden cardiac death following AMI. To predict the severity and the prognosis of an AMI, changes in hemodynamics, ECG and myocardial enzymology indexes are usually observed in the clinic (17).
The structure, size and coronary circulation of the swine heart are similar to that of humans. It is very similar to pathological changes of AMI in humans to block the swine coronary artery branches to induce myocardial infarction, and this has significant implications for research on the pathological physiology and the treatment of AMI. Since there are few branches of the swine coronary artery, it is hard to establish collateral circulation. A swine’s cardiac conduction system also has a poor tolerance to ischemia and hypoxia. Therefore, large infarction area and various malignant arrhythmias can occur easily when the main coronary arteries, especially LAD, are occluded (18). The occlusion of LAD not only leads to massive myocardial ischemia and necrosis, but also influences the cardiac conduction system. Cardiac cell membrane potential decreases and creates the conduction abnormality such as VT and VF, which have led to extremely high mortality rates (19,20). The main reasons for VF include formation of the turn-back ring following acute myocardial ischemia and an increase in automaticity as the base of ventricular arrhythmia, and the low threshold of the ventricular fibrillation in swine, which is easily induced by ischemia and reperfusion (21).
In the present study, an AMI model was created by occluding the bottom-third-site or the middle-site of LAD through balloon angioplasty, suggesting that VT incidence was not statistically different between the two infarction groups. However, the incidence of VF in the middle-site-blocked group was significantly higher than that of the bottom-third-blocked group. This offers a good reference for further studies on prediction indexes of malignant arrhythmia after AMI occurrence.
A previous study showed that QT interval and QTc interval prolongation after AMI can lead to a longer period of phase ventricular vulnerability, followed by an increased susceptibility to rapid ventricular arrhythmia, as well as VT and VF (22). The present study showed that, compared with the control group, QTc interval was significantly prolonged in the Bottom-third-blocked LAD group and the middle-site-blocked group. QT interval extension in the middle-site-blocked LAD group was significantly different compared with that in the control group. QTc interval in the middle-site-blocked LAD group was significantly longer than that in bottom-third-blocked LAD group. Therefore, QTc interval was more sensitive than QT interval after AMI which could result a significantly longer QTc interval. The longer of QTc interval, the more likely to induce malignant arrhythmia (23,24). For those who have a marked extension of QTc interval, more attention should be paid to the possible occurrence of VT and VF. Prolonged QTc interval might be perceived as an independent prediction factor to assess the AMI prognosis.
The location of AMI is correlated with blood supply area of coronary artery branches. A large area infarction caused by LAD occlusion is usually accompanied by shock, left ventricular dysfunction, severe hemodynamic disorder and ventricular arrhythmia. This would lead to an increase in left ventricular end diastolic pressure and pulmonary venous pressure, and to pulmonary interstitial and alveolar edema, decrease in lung compliance and in alveolar ventilation volume, resulting in disorders of ventilation and blood flow, abnormality of diffusion function, the decrease of PaO2 and in the increase of PaCO2 (25). This study indicated that serious hemodynamic disorder existed in both groups after the AMI model establishment with significant MAP and LVEF decrease. MAP in the middle-site-blocked LAD group was significantly lower than that in bottom-third-blocked-LAD group. In addition, compared with that before model establishment, PaO2 decreased significantly while PaCO2 had a marked increase. Consequently, hemodynamic and blood gas analyses may, to some extent, reflect the AMI severity and its prognosis.
AMI affects the heart by exerting direct damage on the myocardial cells, which can be assessed by the serum myocardial enzymes test. In this study, compared with control group, Mb, CK-MB, LDH, AST and cTnT were significantly higher in the Bottom-third-blocked LAD group and the middle-site-blocked group while these indexes were not statistically different between the two AMI groups. This suggested severe myocardial damage brought by AMI whereas the difference in occlusion positions had no significant influence on the change of myocardial enzymology indexes. Necrosis of cardiac cells induced by AMI can lead to the aggregate release of myocardial enzymes, which are later flushed from the infarcted myocardium into blood because of the flushing effect of the opened epicardial vessel, resulting in an early rapid rise in enzyme concentration (26). Myocardial enzyme index was recorded 4 h after AMI. The myocardial enzyme level of this study may not the maximal, and also the rise of myocardial enzyme level might not reflect the infarction area.
Conclusions
In conclusion, LAD occlusion positions affected the occurrence of malignant arrhythmia in an AMI model established by blocking swine LAD through balloon angioplasty. The changes of hemodynamic, ECG, myocardial enzymology and artery blood gas indexes may help us to predict the severity and prognosis of AMI. Since the present study is based on animal experiments, it may not fully reflect the pathophysiological characteristics of clinical patients. Large-scale and multi-center studies of clinical application are needed to confirm the results and to provide more valuable evaluating indicators for risk stratifications of early AMI patients.
Acknowledgements
Grant support: This work was supported by the National Natural Science Foundation of China (Grant No. 81372035, 81170160), the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, Jx10231081), the Foundation of the Health Department of Jiangsu Province (No. H201301), the Six Talents Peak Project of Jiangsu Province (Grant No. 2013WSN035, DG216D5044), the Open Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. SKLNMKF201311, 201408).
Disclosure: The authors declare no conflict of interest.
References
- Nikus KC, Eskola MJ. Electrocardiogram patterns in acute left main coronary artery occlusion. J Electrocardiol 2008;41:626-9. [PubMed]
- Vasudevan K, Manjunath CN, Srinivas KH, et al. Electrocardiographic localization of the occlusion site in left anterior descending coronary artery in acute anterior myocardial infarction. Indian Heart J 2004;56:315-9. [PubMed]
- Varriale P, Leonardi M. Polymorphic ventricular tachycardia in the coronary care unit. Heart Lung 2006;35:283-9. [PubMed]
- Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation 2001;104:2465-70. [PubMed]
- Eldar M, Ohad D, Bor A, et al. A closed-chest pig model of sustained ventricular tachycardia. Pacing Clin Electrophysiol 1994;17:1603-9. [PubMed]
- Ewy GA, Zuercher M, Hilwig RW, et al. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation 2007;116:2525-30. [PubMed]
- Mader TJ, Kellogg AR, Walterscheid JK, et al. A randomized comparison of cardiocerebral and cardiopulmonary resuscitation using a swine model of prolonged ventricular fibrillation. Resuscitation 2010;81:596-602. [PubMed]
- Cheng L, Xiao L. Pig induced pluripotent stem cells: a new resource for generating genetically modified pigs. Regen Med 2009;4:787-9. [PubMed]
- Li X, Zhang F, Song G, et al. Intramyocardial Injection of Pig Pluripotent Stem Cells Improves Left Ventricular Function and Perfusion: A Study in a Porcine Model of Acute Myocardial Infarction. PLoS One 2013;8:e66688. [PubMed]
- Chen Y, Shao DB, Zhang FX, et al. Establishment and evaluation of a swine model of acute myocardial infarction and reperfusion-ventricular fibrillation-cardiac arrest using the interventional technique. J Chin Med Assoc 2013;76:491-6. [PubMed]
- Wessler B, Madias C, Pandian N, et al. Short-term effects of ketamine and isoflurane on left ventricular ejection fraction in an experimental Swine model. ISRN Cardiol 2011;2011:582658.
- Krombach GA, Kinzel S, Mahnken AH, et al. Minimally invasive close-chest method for creating reperfused or occlusive myocardial infarction in swine. Invest Radiol 2005;40:14-8. [PubMed]
- Suzuki Y, Lyons JK, Yeung AC, et al. In vivo porcine model of reperfused myocardial infarction: in situ double staining to measure precise infarct area/area at risk. Catheter Cardiovasc Interv 2008;71:100-7. [PubMed]
- Buecker A, Katoh M, Krombach GA, et al. A feasibility study of contrast enhancement of acute myocardial infarction in multislice computed tomography: comparison with magnetic resonance imaging and gross morphology in pigs. Invest Radiol 2005;40:700-4. [PubMed]
- Yim NY, Kim YH, Choi S, et al. Multidetector-row computed tomographic evaluation of myocardial perfusion in reperfused chronic myocardial infarction: value of color-coded perfusion map in a porcine model. Int J Cardiovasc Imaging 2009;25 Suppl 1:65-74. [PubMed]
- Walker MJ, Curtis MJ, Hearse DJ, et al. The Lambeth Conventions: guidelines for the study of arrhythmias in ischaemia infarction, and reperfusion. Cardiovasc Res 1988;22:447-55. [PubMed]
- Anyukhovsky EP, Sosunov EA, Kryukova YN, et al. Expression of skeletal muscle sodium channel (Nav1.4) or connexin32 prevents reperfusion arrhythmias in murine heart. Cardiovasc Res 2011;89:41-50. [PubMed]
- Li XD, Yang YJ, Geng YJ, et al. Phosphorylation of endothelial NOS contributes to simvastatin protection against myocardial no-reflow and infarction in reperfused swine hearts: partially via the PKA signaling pathway. Acta Pharmacol Sin 2012;33:879-87. [PubMed]
- Odenstedt J, Mansson C, Jansson SO, et al. Endocardial electromechanical mapping in a porcine acute infarct and reperfusion model evaluating the extent of myocardial ischemia. J Invasive Cardiol 2003;15:497-501. [PubMed]
- Wrobleski D, Houghtaling C, Josephson ME, et al. Use of electrogram characteristics during sinus rhythm to delineate the endocardial scar in a porcine model of healed myocardial infarction. J Cardiovasc Electrophysiol 2003;14:524-9. [PubMed]
- Reddy VY, Wrobleski D, Houghtaling C, et al. Combined epicardial and endocardial electroanatomic mapping in a porcine model of healed myocardial infarction. Circulation 2003;107:3236-42. [PubMed]
- Bonnemeier H, Hartmann F, Wiegand UK, et al. Course and prognostic implications of QT interval and QT interval variability after primary coronary angioplasty in acute myocardial infarction. J Am Coll Cardiol 2001;37:44-50. [PubMed]
- Ueda H, Nakayama Y, Tsumura K, et al. Intravenous nicorandil can reduce the occurrence of ventricular fibrillation and QT dispersion in patients with successful coronary angioplasty in acute myocardial infarction. Can J Cardiol 2004;20:625-9. [PubMed]
- Chander S, Kumar R, Jorapur V, et al. Effect of mechanical coronary reperfusion on QT dispersion in acute coronary syndrome. Indian Heart J 2005;57:233-6. [PubMed]
- Indik JH, Donnerstein RL, Hilwig RW, et al. The influence of myocardial substrate on ventricular fibrillation waveform: a swine model of acute and postmyocardial infarction. Crit Care Med 2008;36:2136-42. [PubMed]
- Xue M, Yin H, Zhang L, et al. Dynamic expression of the main related indicators of thrombosis, inflammatory reaction and tissue damage in a rat model of myocardial infarction. Mol Med Rep 2011;4:693-6. [PubMed]


