
The role of diet in the development and management of gastroesophageal reflux disease: why we feel the burn
Introduction
Gastroesophageal reflux disease (GERD) occurs when troublesome symptoms and/or mucosal disease develop in the setting of refluxed stomach contents (1). Most commonly, this is characterized by the presence of burning mid-sternal chest pain, regurgitation of fluid or food, or development of esophageal inflammation that may lead to swallowing dysfunction (2). Additionally, patients may experience extraesophageal manifestations including cough, bronchospasms, and hoarseness (3). Its incidence is high in the general population, estimated to affect up to a third of people worldwide (4). Risk factors for symptom development include central adiposity, smoking, and genetic predisposition (5). Acid suppressive therapy and lifestyle modifications are first-line treatment options, with the latter increasing in popularity due to potential side effects of pharmacotherapy (6). Lifestyle interventions commonly recommended include alterations in diet, weight management, smoking cessation, and head of bed elevation while recumbent (5). Although dietary manipulation is commonly employed in clinical practice, data is conflicting on definitive recommendations (7). The following is a review of the data implicating diet in GERD with an emphasis on the effect food components have on pathophysiology and management.
Pathophysiology of GERD
Mechanistically, GERD and associated complications develop when esophageal mucosa is abnormally exposed to refluxed stomach contents. This may be in the setting of incompetence of the gastroesophageal barrier, inadequate clearance of refluxed fluid, or alterations in refluxed fluid content (8). Repeated exposure leads to alterations in mucosal integrity and cellular composition. This can lead to the development of inflammation, scar tissue and changes in visceral sensitivity. Resultant complications include esophageal and extraesophageal symptoms, stricture development, dysmotility, and/or carcinogenesis (2). Esophageal exposure to gastric contents may be altered by dietary manipulation.
Gastroesophageal barrier
The gastroesophageal barrier includes intrinsic tone of the lower esophageal sphincter (LES), external pressure via the crural diaphragm, and an intact angle of His (3). Of these components, LES pressure is the only mechanism affected by dietary intake and therefore will be the focus of the proceeding discussion. Tone of the LES is dependent on neural, hormonal, and paracrine factors which maintain intrinsic contraction. Innervation is provided by the myenteric plexus, which has both excitatory and inhibitory neuronal components. Excitatory neurons are controlled by acetylcholine and substance P while inhibitory neurons use vasoactive intestinal peptide and nitric oxide (9). Release of neurohormonal stimulants is affected by oral intake and altered based on caloric density and chemical composition of ingested food (10).
Disruption of the gastroesophageal barrier also occurs during transient lower esophageal sphincter relaxations (TLESRs) during which the LES relaxes to release excess gas from the stomach. TLESRs occur in the setting of gastric distention, which stimulates a vasovagal reflex and LES relaxation (11). Gastric distention may be secondary to ingestion of food or air and is exacerbated by alterations in gastric motility, which may be affected by type of oral intake and medications.
Inadequate clearance of refluxed gastric fluid
In addition to the LES which serves as an anatomic barrier, esophageal mucosa is protected from damage by minimizing the duration of refluxed stomach contents in the esophagus. This is facilitated by peristalsis, which is involuntary contraction of the esophagus that propels contents proximally. Amplitude of contractions is dependent on sensory feedback from the esophagus. Therefore, large boluses of food, or those that have increased viscosity may slow down contractions. Increased intra-abdominal pressure or retainment of food in the esophageal or stomach also serve as inhibitory mechanisms (12).
Alterations in refluxed fluid
Refluxed stomach fluid consists of several esophageal mucosal irritants including gastric acid, digestive enzymes, and bile salts. Secretion of these digestive components is dependent on dietary intake and may be altered with changes in nutritional composition (13). Undigested food particles may additionally be refluxed into the esophagus, with varying effects on underlying mucosa.
Overview of dietary composition and metabolism
In addition to understanding the pathophysiology implicated in GERD development, an understanding of nutritional composition is helpful when discussing dietary manipulation. The human diet is composed of three main types of macronutrients, which have differing caloric density and biochemical compositions. These dietary components are broken down to produce energy and support cellular metabolism. The three macronutrients include carbohydrates, fats, and proteins.
Carbohydrates
Carbohydrates traditionally make up the majority of ingested calories and serve as an important source of glucose molecules for energy. They are classified based on chemical complexity, divided into three categories: monosaccharides, oligosaccharides, and polysaccharides. Monosaccharides consist of a single sugar molecule and therefore are also referred to as “simple sugars”. Examples of monosaccharides include fructose, galactose, and glucose (14). Simple sugars are metabolized quickly in the body, rapidly raising blood sugar levels and producing an osmotic effect in the gastrointestinal tract. (9). This contrasts with more complex carbohydrates including oligosaccharides and polysaccharides which are comprised of multiple linked sugar molecules. Examples of oligosaccharides include lactose, maltose, and sucrose (13). Polysaccharides are longer in length and include the common storage forms of glucose, starch and fiber. Some complex carbohydrates can be digested utilizing endogenous enzymes while others are not metabolically available to human hosts (15). The former includes starch, a storage form of alpha-linked glucose molecules which can be broken down by salivary and pancreatic secretions. The latter consists of fiber, which is beta-linked and inaccessible to digestive enzymes (13).
Fats
Fat is the most calorically dense macronutrient, with every gram of fat equaling nine kilocalories of energy (as opposed to the four kilocalories of energy found in carbohydrates and proteins). Like carbohydrates, fats are classified by chemical composition, which looks at type of bonds found between carbon molecules and the length of the carbon molecule chain. The three types of fats include saturated fats, which contain no carbon-carbon double bonds, monosaturated fats, which contain one double bond, and polyunsaturated fats, which contain more than one double bond (13). The chemical composition of bonds alters how easily a fat can be metabolized. Additionally, the length of the carbon chain determines whether a transporter is needed to absorb nutrients across the small bowel mucosa, with shorter chain fats directly absorbed (16).
Proteins
Proteins are comprised of amino acids and are the main source of nitrogen in the body. They are categorized based on their amino acid profile, which determine whether a protein is complete (contains all essential amino acids which are not endogenously synthesized by the body) or incomplete. Their digestion occurs in the small bowel with the aid of pancreatic and intestinal enzymes (15).
Diet and GERD
Dietary therapy for GERD is commonly prescribed, though data supporting specific interventions is variable. Earlier dietary studies focused on analyzing types of foods or beverages in terms of their effect on GERD pathophysiology and symptoms. More recently, dietary patterns including macronutrient composition and eating behavior have been assessed, which may be a more practical approach for patients (Table 1).
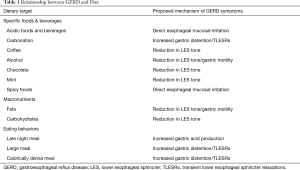
Full table
Elimination diets
Eliminating entire food or beverage categories is a common practice in primary care and gastroenterology clinics and is both intuitive and easy to convey to patients. This approach should be personalized to patient response.
Beverages
Avoiding specific beverage types or attributes is commonly recommended for GERD management, however most of these recommendations are based on limited data. For instance, acidic beverages are often hypothesized to worsen GERD and it has been shown that physiologically acidic fluids drop the pH of refluxed stomach contents and increase esophageal clearance time (17). In practice, correlation of symptoms is less clear. For example, a survey of 394 patients with heartburn noted a weak correlation between titratable acid content of popular beverages and symptoms (r=0.59, P<0.01). However some beverages with high acidity did not induce symptoms (i.e., prune juice) and others with low acidity did (i.e., tomato juice) indicating other factors may play a role (18). Similar incongruencies between physiology and symptoms exist for carbonated beverages. For instance, carbonation has been shown to transiently alter intra-abdominal pressure and TLESR frequency, leading to reduction in LES tone (19). However, physiological changes with ingestion are transient and have not been correlated with GERD symptoms as reported by a systemic review of 17 studies (20). Similarly, early literature suggests coffee decreases LES tone after ingestion (21). But despite this transient effect, the most recently published meta-analysis showed no discernable association between coffee intake, GERD symptoms, or mucosal disease. This lack of association remained on subgroup analysis of low intake users (<4 cups/day) vs. high-intake (>4 cups/day) indicating absence of symptom correlation even with large ingestions (22).
Data linking alcoholic beverages and GERD is more mixed. A small study in 25 healthy volunteers showed increase in reflux episodes after beer or wine ingestion in comparison to water (23). This does not correlate with findings in larger cohort studies, such as a case-controlled evaluation of 3,153 patients with GERD in comparison to 40,210 patients without that showed no relationship (24). Similar findings have been reported in an American population as well as a large cross-sectional analysis of mostly European and North American patients (24,25). In contrast, the most recently published meta-analysis of observational and case-controlled studies reported positive correlation between alcohol use and GERD symptoms (OR =1.48, 95% CI: 1.31–1.67), with a greater effect noted on subgroup analysis of patients with reflux esophagitis on upper endoscopy (OR =1.78, 95% CI: 1.56–2.03) (26). This meta-analysis primarily featured newer studies conducted in Asia indicating genetic and demographic factors may play a role in pathophysiology of disease.
Foods and spices
In addition to implicated beverages, patients are often counseled to avoid certain foods and spices. As in beverages, these recommendations are based on limited data and need to be personalized. For instance, chocolate, which contains both caffeine as well as cacao, induces LES relaxation (27). But this finding does not correlate with symptom frequency, even with those who eat large amounts of chocolate per day as per a study of 500 Italian adults (7). Mint is also a commonly reported dietary trigger for patients, though may only affect a small percentage of those with GERD. This was demonstrated by several older studies that demonstrated physiological basis by correlating peppermint with rapid LES relaxation but showed only a minority of patients (6/20) had exacerbation of symptoms with high levels of ingestion (28). In contrast, spicy foods do not induce any physiological changes, but may act as a direct irritant to esophageal mucosa. This was found to mimic classic heartburn features as reported by a study of 16 healthy volunteers indicating those that are sensitive to spice may benefit from avoiding it (29).
Macronutrient composition and dietary patterns
Considering the variability of data supporting specific elimination diets, recent research studies have focused on eating patterns and macronutrient manipulation. These approaches provide broader recommendations for dietary alteration.
Fats
High fat diets, especially those which include fried or greasy foods, are hypothesized to worsen GERD symptoms. Much like specific elimination diets, however, data remains inconsistent and personalized recommendations for patients are necessary. As previously discussed, fat is calorically dense, and digestion often requires secretion of potential esophageal irritants (i.e., bile salts) and neurohormonal mediators of LES tone (i.e., Cholecystokinin). Physiological studies have examined this proposed relationship, with mixed results. For example, in a double-blinded randomized controlled study of 12 healthy volunteers, isocaloric delivery of a low-fat meal (10% of calories) in comparison to a high fat meal (50% of calories) did not affect mean LES pressures, frequency of TLESRs, or number of reflux episodes (30). This contrasts with earlier studies that report increased esophageal acid exposure time and alterations in LES pressures after fat ingestion (31,32). Several population studies have favored correlation of fat intake with GERD symptoms, however confounding factors include total caloric intake and BMI of study participants (33,34). The largest population cohort study investigating the question, which utilized the National Health and Nutrition Examination Survey and investigated over 12,000 patients, found no correlation between dietary fat intake and GERD symptoms (35).
Notably no study has distinguished between fat type [i.e., saturated vs. unsaturated or medium chain triglyceride (MCT) vs. long chain triglyceride (LCT)], which may have clinical importance. For example, a controlled study of 15 healthy volunteers receiving duodenal lipid infusions noted increase in gastric volume and CCK release with LCT administration as opposed to MCTs (36). This relationship between fat type and GERD symptoms will need to be explored in future research.
Carbohydrates
In addition to reduction of fat in the diet, modulation of carbohydrate intake has been explored with more conclusive findings. As previously discussed, carbohydrates are categorized based on chemical composition and carbon structure, which affects gastrointestinal processing. Ingestion of disaccharides and starches are of most interest, as these carbohydrates are only partially absorbed in the small bowel, later undergoing fermentation by colonic bacteria. This fermentation process has been shown to induce neurohormonal release and LES relaxation which may lead to heartburn (37). Correlation between GERD symptoms, reflux episodes, and carbohydrate ingestion has been explored through several recent studies. For instance, an analysis of 12 patients with GERD randomized to a high carbohydrate (178.8 g) vs. low carbohydrate (84.8 g) liquid meal showed statistically significant differences between impedance tracings in terms of total esophageal acid exposure time and number of reflux episodes (38). Another small prospective study linked a very low carbohydrate diet (20 g/day) with reduction in esophageal acid exposure time (39). These results were replicated in a larger study investigating 144 obese women with GERD who had symptom resolution after alterations in starch ingestion and simple sugar intake (40). Most recently, a prospective study of 130 patients with reflux who followed a low glycemic diet for a two-week period found statistically significant improvement in symptoms, although notably the patients also lost weight which could have confounded outcomes (41).
Carbohydrate type is important in studying the link between GERD and ingestion of sugars. While monosaccharides and starches have been linked to increase in symptomatology, the opposite has been found in relation to fiber intake. For instance, a survey of 371 employees at a large medical center noted an inverse relationship between heartburn symptoms and fiber intake even after correcting for confounding variables (33). This finding has been replicated in small, prospective trials. For example, 45 GERD patients taking a soluble fiber preparation for two weeks noted equal improvement in their heartburn symptoms as a group prescribed an antacid (42). Similarly, 36 patients previously consuming a low fiber diet (<20 g/day) and later given three times daily Psyllium were found to have a reduction in both symptoms and mean reflux episodes by pH impendence testing (43). The mechanism by which fiber improves heartburn is unknown.
Proteins
Few studies have examined the role of dietary protein in relation to GERD despite early research linking protein intake with increase in LES pressures (28). A large survey of medical center employees which previously linked high fiber intake with lower risk of symptoms also reported no difference in protein intake between groups (33). Further research is warranted.
Eating patterns
In addition to specific elimination diets and manipulation of the macronutrient content, eating patterns are implicated in GERD. This includes avoidance of late-night eating and reduction in overall size and caloric density of meals. The principle of early meal times is based on small studies that have correlated early dinners with sustained increase in nocturnal pH and reduction in supine reflux episodes (44-46). This finding is thought to be secondary to increased gastric acid secretion with food intake, as well as physical presence of food in the stomach when a patient is supine (47). Typically it can take up to 4 hours for 90% of a solid meal to move forward out of the stomach.
Not all prospective trials have shown these effects. For instance, a study of 20 volunteers with reflux showed no difference between symptom frequencies after an isocaloric meal given at two hours vs. four hours before bedtime (48). However, since most prospective analyses have indicated benefit (especially in comparison between early and late meals), this can be considered an option for patients with symptomatic disease.
As for the size and caloric density of meals, this has also been studied in small trials as well as larger survey-based studies. For example, a trial including 13 healthy volunteers ingesting varying levels of calories at meal time found increased consumption of calories was linked to increased intraesophageal acid exposure time on pH impendence testing (49). A large cross-sectional analysis of nearly 12,000 patients in Korea confirmed a similarly positive relationship between non-erosive reflux disease and total caloric intake daily, though notably did not stratify by calories per meal and may have been confounded by BMI status (50).
Specific diets
Specific diets which reduce common triggers of GERD including simple sugars and fats and increase fiber intake may be offered to patients looking for more comprehensive dietary planning. For example, the Mediterranean Diet, which includes high intake of fruits, vegetables, whole grains, and unsaturated fats, was recently studied in a large cohort of Albanian patients. When corrected for demographic and lifestyle factors including eating habits, those following a traditional Mediterranean diet had lower reported incidence of GERD symptoms (51). A follow up retrospective study of patients receiving standard acid suppression therapy vs. those on a Mediterranean diet and other common lifestyle alterations found equal efficacy of the two approaches on the reflux symptom index, which is a common survey tool used to assess extra esophageal manifestations of GERD (52).
Conclusions
Although heterogeneity exists in the literature supporting dietary intervention in GERD, common themes have emerged which can guide patient care. Firstly, diets should be individualized for patients based on symptoms, with reintroduction of foods and habits if there is ineffective control of symptoms. Secondly, manipulation of meal size, timing, and overall macronutrient composition appears to be more effective than elimination diets, with an overall emphasis on reduction of meal size, carbohydrate content (especially simple sugars), and late-night eating patterns. Finally, dietary patterns should be additive to an overall anti-reflux plan which includes additional lifestyle modifications such as smoking cessation, weight loss, and head of bed elevation in those with nocturnal symptoms. As patients continue to pursue non-pharmacological options for disease management, well-designed randomized controlled trials are needed to study the effects of dietary therapy on GERD management.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Vakil N, van Zanten SV, Kahrilas P, et al. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Am J Gastroenterol 2006;101:1900-20. [Crossref] [PubMed]
- Spechler SJ. Clinical manifestations and esophageal complications of GERD. Am J Med Sci 2003;326:279-84. [Crossref] [PubMed]
- Vela, MF, Richter, JE, Pandolfino, JE, editors. Practical manual of gastroesophageal reflux disease Portland: Ringgold, Inc; 2013.
- El-Serag HB, Sweet S, Winchester CC, et al. Update on the epidemiology of gastro-oesophageal reflux disease: A systematic review. Gut 2014;63:871-80. [Crossref] [PubMed]
- Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol 2016;14:175. [Crossref] [PubMed]
- Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology 2017;153:35-48. [Crossref] [PubMed]
- Kaltenbach T, Crockett S, Gerson LB. Are lifestyle measures effective in patients with gastroesophageal reflux disease? An evidence-based approach. Arch Intern Med 2006;166:965-71. [Crossref] [PubMed]
- De Giorgi F, Palmiero M, Esposito I, et al. Pathophysiology of gastro-oesophageal reflux disease. Acta Otorhinolaryngol Ital 2006;26:241. [PubMed]
- Esatbeyoglu T, Rimbach G. Canthaxanthin: From molecule to function. Mol Nutr Food Res 2017;61. [Crossref] [PubMed]
- Lentle, RG, Janseen, PW. The Physical Processes of Digestion. New York: Springer; 2011.
- Kim HI, Hong SJ, Han JP, et al. Specific Movement of Esophagus During Transient Lower Esophageal Sphincter Relaxation in Gastroesophageal Reflux Disease. J Neurogastroenterol Motil 2013;19:332-7. [Crossref] [PubMed]
- Shaker R, Belafsky PC, Postma GN, Easterling C, editors. Principles of Deglutition. New York: Springer 2013.
- MacFarlane NG. Digestion and absorption. Anesth Int Care Med 2018;19:125-7. [Crossref]
- Dobbing J, editor. Dietary Starches and Sugars in Man: A Comparison. London: Springer; 1989.
- Gray GM, Cooper HL. Protein Digestion and Absorption. Gastroenterology 1971;61:535-44. [Crossref] [PubMed]
- Griffin BA. Lipid Metabolism. Surgery 2013;31:267-72.
- Gomes DC, Dantas RO. Acidic and neutral liquid ingestion in patients with gastroesophageal reflux disease. Arq Gastroenterol 2014;51:217-20. [Crossref] [PubMed]
- Feldman M, Barnett C. Relationships between the acidity and osmolality of popular beverages and reported postprandial heartburn. Gastroenterology 1995;108:125-31. [Crossref] [PubMed]
- Cuomo R, Sarnelli G., Savarese MF, et al. Carbonated beverages and gastrointestinal system: Between myth and reality. Nutr Metab Cardiovasc Dis 2009;19:683-9. [Crossref] [PubMed]
- Johnson T, Gerson L, Hershcovici T, et al. Systematic review: The effects of carbonated beverages on gastro-oesophageal reflux disease. Aliment Pharmacol Ther 2010;31:607-14. [Crossref] [PubMed]
- Zhang Y, Chen S. Effect of coffee on gastroesophageal reflux disease. Food Sci Tech Res 2013;19:1-6. [Crossref]
- Kim J, Oh S, Myung S, Kwon H, et al. Association between coffee intake and gastroesophageal reflux disease: A meta‐analysis. Dis Esophagus 2014;27:311-7. [Crossref] [PubMed]
- Hamoui N, Lord RV, Hagen JA, et al. Response of the lower esophageal sphincter to gastric distention by carbonated beverages. J Gastrointest Surg 2006;10:870-7. [Crossref] [PubMed]
- Nilsson M, Johnsen R, Ye W, et al. Prevalence of gastro-oesophageal reflux symptoms and the influence of age and sex. Scand J Gastroenterol 2004;39:1040-5. [Crossref] [PubMed]
- Haycox A., Einarson T., Eggleston A. The health economic impact of upper gastrointestinal symptoms in the general population: Results from the domestic/international gastroenterology surveillance study (DIGEST). Scand J Gastroenterol Suppl 1999;231:38-47. [Crossref] [PubMed]
- Pan J, Cen L, Chen W, et al. Alcohol consumption and the risk of gastroesophageal reflux disease: A systematic review and meta-analysis. Alcohol Alcohol 2019;54:62-9. [Crossref] [PubMed]
- Babka JC, Castell DO. On the genesis of heartburn. The effects of specific foods on the lower esophageal sphincter. Amer J Dig Dis 1973;18:391-7. [Crossref] [PubMed]
- Benamouzig R, Airinei G. Diet and reflux. J Clin Gastroenterol 2007;41 Suppl 2:S71. [Crossref]
- Yeoh KG, Ky Ho, Guan R, et al. How does chili cause upper gastrointestinal symptoms? A correlation study with esophageal mucosal sensitivity and esophageal motility. J Clin Gastroenterol 1995;21:87-90. [Crossref] [PubMed]
- Pehl C, Waizenhoefer A, Wendl B, et al. Effect of low and high fat meals on lower esophageal sphincter motility and gastroesophageal reflux in healthy subjects. Am J Gastroenterol 1999;94:1192-6. [Crossref] [PubMed]
- Becker DJ, Sinclair J, Castell DO. A comparison of high and low-fat meals on postprandial esophageal acid exposure. Am J Gastroenterol 1989;84:782. [PubMed]
- Iwakiri K, Kobayashi M, Kotoyori M, et al. Relationship between postprandial esophageal acid exposure and meal volume and fat content. Dig Dis Sci 1996;41:926-30. [Crossref] [PubMed]
- El-Serag HB, Satia JA, Rabeneck L. Dietary intake and the risk of gastro-oesophageal reflux disease: A cross sectional study in volunteers. Gut 2005;54:11-7. [Crossref] [PubMed]
- Fox M, Barr C, Nolan S, et al. The effects of dietary fat and calorie density on esophageal acid exposure and reflux symptoms. Clin Gastroenterol Hepatol 2007;5:439-44. [Crossref] [PubMed]
- Ruhl CE, Everhart JE. Overweight, but not high dietary fat intake, increases risk of gastroesophageal reflux disease hospitalization: The NHANES I epidemiologic followup study. Ann Epidemiol 1999;9:424-35. [Crossref] [PubMed]
- Feinle C, Rades T, Otto B, et al. Fat digestion modulates gastrointestinal sensations induced by gastric distention and duodenal lipid in humans. Gastroenterology 2001;120:1100-7. [Crossref] [PubMed]
- Piche T, des Varannes SB, Sacher-Huvelin S, et al. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology 2003;124:894-902. [Crossref] [PubMed]
- Wu KL, Kuo CM, Yao CC, et al. The effect of dietary carbohydrate on gastroesophageal reflux disease. J Formos Med Assoc 2018;117:973-8. [Crossref] [PubMed]
- Austin GL, Thiny M, Westman E, et al. A very low-carbohydrate diet improves gastroesophageal reflux and its symptoms. Dig Dis Sci 2006;51:1307-12. [Crossref] [PubMed]
- Pointer SD, Rickstrew J, Slaughter JC, et al. Dietary carbohydrate intake, insulin resistance and gastro-oesophageal reflux disease: a pilot study in European- and African-American obese women. Aliment Pharmacol Ther 2016;44:976-88. [Crossref] [PubMed]
- Langella C, Naviglio D, Marino M, et al. New food approaches to reduce and/or eliminate increased gastric acidity related to gastroesophageal pathologies. Nutrition 2018;54:26-32. [Crossref] [PubMed]
- DiSilvestro RA, Verbruggen MA, Offutt EJ. Anti-heartburn effects of a fenugreek fiber product. Phytother Res 2011;25:88-91. [Crossref] [PubMed]
- Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol 2018;24:2291-9. [Crossref] [PubMed]
- Fujiwara Y, Machida A, Watanabe Y, et al. Association between dinner-to-bed time and gastro-esophageal reflux disease. Am J Gastroenterol 2005;100:2633-6. [Crossref] [PubMed]
- Piesman M, Hwang I, Maydonovitch C, et al. Nocturnal reflux episodes following the administration of a standardized meal. Does timing matter? Am J Gastroenterol 2007;102:2128-34. [Crossref] [PubMed]
- Duroux P, Bauerfiend P, Emde C, et al. Early dinner reduces nocturnal gastric acidity. Gut 1989;30:1063. [Crossref] [PubMed]
- Hunt JN, Stubbs DF. The Volume and Energy Content of Meals as Determinants of Gastric Emptying. J Physiol 1975;245:209-25. [Crossref] [PubMed]
- Orr WC. Sleep-related gastro-oesophageal reflux: Provocation with a late evening meal and treatment with acid suppression. Aliment Pharmacol Ther 1998;12:1033-8. [Crossref] [PubMed]
- Colombo P, Mangano M, Bianchi PA, et al. Effect of calories and fat on postprandial gastro-oesophageal reflux. Scand J Gastroenterol 2002;37:3-5. [Crossref] [PubMed]
- Nam SY, Park B, Cho Y, et al. Different effects of dietary factors on reflux esophagitis and non-erosive reflux disease in 11,690 Korean subjects. J Gastroenterol 2017;52:818-29. [Crossref] [PubMed]
- Mone I, Kraja B, Bregu A, et al. Adherence to a predominantly Mediterranean diet decreases the risk of gastroesophageal reflux disease: a cross-sectional study in a South Eastern European population. Dis Esophagus 2016;29:794-800. [Crossref] [PubMed]
- Zalvan CH, Hu S, Greenberg B, et al. A Comparison of Alkaline Water and Mediterranean Diet vs Proton Pump Inhibition for Treatment of Laryngopharyngeal Reflux. JAMA Otolaryngol Head Neck Surg 2017;143:1023-9. [Crossref] [PubMed]


