
A nomogram prediction model for recurrent laryngeal nerve lymph node metastasis in thoracic oesophageal squamous cell carcinoma
Introduction
Oesophageal carcinoma is the 8th most common cancer worldwide and the 6th leading cause of cancer-related death, and the 5-year survival rate is only 15–25% worldwide (1). In China (2), the estimated number of new oesophageal cancer cases and deaths per year are 477.9 and 375.0 thousand, respectively.
Oesophagectomy with 3-field lymphadenectomy increases the overall 5-year survival rate to 40–55.6% (3-7). However, this technique also increases the complexity of the operation and complications (5-9), especially the dissection of lymph nodes (LNs) around the recurrent laryngeal nerve (RLN) (10-12). Furthermore, not all patients can benefit from the dissection of the RLN LNs (11,12). Prediction of RLN LN metastasis will provide a reference for planning the treatment regimen, selecting the surgical approach and planning for intraoperative LN dissection. Therefore, we retrospectively analysed the clinicopathological data of 265 consecutive oesophageal squamous cell carcinoma (ESCC) patients who underwent oesophagectomy at the General Hospital of the Jinan Military Area and the Second Affiliated Hospital of Shandong University to formulate a statistical model to accurately predict RLN LN metastasis in thoracic ESCC patients.
Methods
Study cohort
In this retrospective study, a total of 542 consecutive patients who underwent curative oesophagectomy for oesophageal carcinoma at the General Hospital of the Jinan Military Area and the Second Affiliated Hospital of Shandong University between January 2015 and June 2018 were studied. The clinicopathological data of 265 patients who met the following conditions were collected.
The inclusion criteria were as follows: (I) preoperative or postoperative pathology proven to be primary ESCC; (II) no distant metastasis found on thoraco-abdominal enhanced computed tomography (CT), cervical ultrasonography, electronic gastroscopy, endoscopic ultrasound and (or) whole-body positron emission tomography CT (PET-CT); (III) underwent thoracoscopic-assisted tri-incisional oesophagectomy performed with two- or three-field lymphadenectomy and complete dissection of the RLN LNs; and (IV) complete clinicopathological data. The exclusion criteria were as follows: (I) patients who accepted neoadjuvant chemotherapy or radiotherapy (cases treated by neoadjuvant therapy usually come into pathological changes that cannot be well elucidated by this issue); (II) cases that were combined with other malignant tumours; (III) pathological results revealing other cellular components in addition to squamous cell carcinoma; (IV) patients in whom RLN LN dissection was not performed (postoperative pathological evaluation showing that the number of bilateral RLN LNs was zero); or (V) incomplete clinicopathological data.
Surgical methods
All patients underwent subtotal oesophageal excision by a tri-incisional approach involving thoracoscopic resection of the oesophagus and mediastinal lymphadenectomy in a left semi-prone position, followed by laparoscopic mobilization of the stomach and abdominal LN dissection in a supine position and left-sided cervical anastomosis (Figure 1A,B,C). Extensive lymphadenectomy that involved resection of the bilateral upper mediastinal LNs in addition to standard lymphadenectomy was performed during every operation (Figure 1D,E,F). Cervical lymphadenectomy (32 patients underwent 3-field lymphadenectomy, while 233 patients underwent 2-field lymphadenectomy) was only performed in cases of suspicious cervical LN metastasis by cervical ultrasound or PET-CT. The extent of RLN LN dissection and determination of the tumour location were defined in accordance with the Japanese Classification of Esophageal Cancer, 11th edition: parts II and III (13).
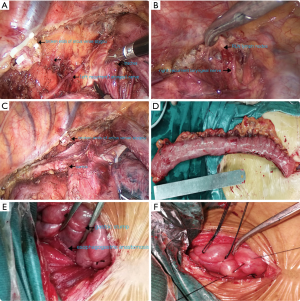
The left RLN LNs were dissected as follows. First, the upper thoracic oesophagus was removed from the oesophageal bed, and the left RLN was dissected from the dorsal side of the aortic arch and moved upward to the left tracheoesophageal groove. LNs and surrounding fatty tissues around the nerve were completely dissected (Figure 1A). The right RLN LNs were dissected as follows: we first exposed the right subclavian artery by removing the mediastinal pleura above the azygos arch. The right RLN originates from the vagus nerve trunk at the level of the right subclavian artery and extends upward to the lower pole of the thyroid. The LNs together with the surrounding fatty tissues were removed carefully (Figure 1B).
Surgically dissected LNs were classified according to the definition of regional LNs, given individual names or numbers and sent to pathologists. The LNs that were dissected en bloc with the oesophagus were isolated from the specimen before fixation. All of these primary tumour samples were fixed in 10% neutral buffered formalin, embedded in paraffin and stained with haematoxylin and eosin in a routine manner.
Observation index
The following data were recorded: (I) general information for the patients, including gender and age; (II) preoperative examination findings, including measurements of the short-axis diameter of the largest left and right RLN LNs, maximum diameter of the tumour and the tumour location as determined by gastroscopy and CT, with the short-axis diameter of the largest left and right RLN LNs divided into 2 groups by cut-off points of 5 mm (14,15); and (III) intraoperative pathological data, including the degree of differentiation, subcarinal LN (SLN) status, paraoesophageal LN (PLN) status, left and right RLN LN status and total number of resected LNs. Patient and operative characteristics were collected from the medical records, and all pathological characteristics were obtained from the final pathology reports completed by two professional pathologists.
This study was reviewed and approved by the Ethics Committee of the General Hospital of the Jinan Military Area (JN2018012) and the Second Affiliated Hospital of Shandong University (SD20180131). All patients provided written informed consent for their procedures.
Statistical analysis
Statistical analysis was performed with SPSS 19.0 software (SPSS Inc., Chicago, Illinois, USA). Measurement data conforming to a normal distribution are described as the mean ± standard deviation. The risk factors for RLN LN metastasis included deviation. The Levene test was used to judge the homogeneity of variance. Independent sample t-tests (two-sided) were used for inter-group comparisons. Numerical data are expressed in terms of frequencies and percentages and were compared by the Pearson χ2 test (two-sided); Fisher’s exact test was used when the expected frequencies of one or more cells were less than 5. The risk factors for RLN LN metastasis were analysed by univariate analysis, and variables with a P value <0.1 were entered into multivariate logistic regression to formulate a model for predicting RLN LN metastasis. The Hosmer-Lemeshow goodness of fit test and the area under receiver operator characteristic (ROC) curve (AUC) were calculated to evaluate the model. By using R software, we constructed the nomogram according to the results of the logistic regression analysis.
Results (Figures 2-4)

Postoperative complications and lymphatic dissection
There were 265 patients who matched the conditions above, of which 222 (83.8%) were males, and 43 (16.2%) were females, aged from 41 to 68 years old [95% confidence interval (CI): 63.03±1.00]. A total of 258 patients (97.4%) underwent R0 resection, and 7 underwent R1 resection. The overall 30-day mortality rate was 2.3%. Two patients died from aortic rupture, and 2 died from respiratory failure, which resulted in an operation-specific 30-day mortality rate of 1.5%. Postoperative complications occurred in 53 (20.0%) patients. Notably, 13.6% of the patients experienced RLN injuries, of which 19.4% were bilateral.
A total of 6,255 LNs were resected from 265 patients, with 23.60±1.04 [7–58] LNs dissected per patient. The average number of left RLN LNs was 2.34±0.33 [0–16], and the metastatic rate was 15.1%. For the right RLN LNs, the average number of LNs dissected and the metastatic rate were 2.94±0.31 [0–17] and 20.4%, respectively. The total rate of RLN LN metastasis was 28.3%.
Analysis of RLN LN metastasis
Univariate and multivariate analysis of RLN LN metastasis
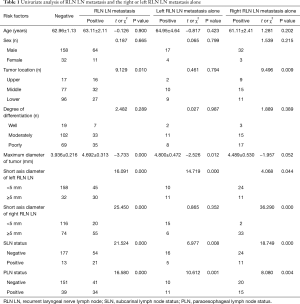
Univariate analysis (Table 1) showed that risk factors for RLN LN metastasis included the short-axis diameter of the left RLN LN, the short-axis diameter of the right RLN LN, maximum tumour diameter, tumour location, SLN status and PLN status. Multivariate logistic regression analysis (Table 2) showed that all six variables listed above were independent predictive factors of RLN LN metastasis.
Full table
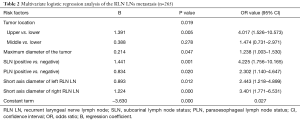
Full table
Evaluation of the fit of the regression model and construction of the nomogram
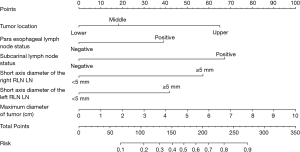
The Hosmer-Lemeshow test calculated that χ2 =4.901, P=0.768. A ROC curve was constructed according to the regression model (Figure 2A), and the area under the ROC curve (AUC) was 0.809 (95% CI: 0.754–0.864). When a predictive probability =0.143 was used as the cut-off value, the sensitivity and specificity of the nomogram for predicting RLN LN metastasis were 94.7% and 43.7%, respectively. The nomogram (Figure 3) constructed according to the regression model included the following six variables: short-axis diameter of the left RLN LN, short-axis diameter of the right RLN LN, maximum tumour diameter, tumour location, SLN status and PLN status.
Analysis of right RLN LN metastasis alone
Multivariate analysis of right RLN LN metastasis alone
Univariate analysis (Table 1) showed that risk factors for right RLN LN metastasis included the short-axis diameter of the left RLN LN, short-axis diameter of the right RLN LN, maximum tumour diameter, tumour location, SLN status and PLN status. Multivariate logistic regression analysis (Table 3) showed that short-axis diameter of the right RLN LN, tumour location, and SLN status were independent predictive factors of right RLN LN metastasis.
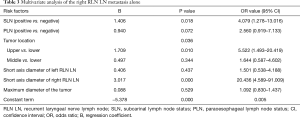
Full table
Evaluation of the fit of the regression model and construction of the nomogram
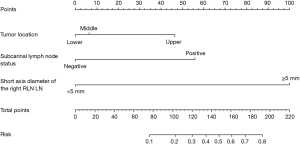
The Hosmer-Lemeshow test calculated that χ2=1.735, P=0.988. A ROC curve was constructed according to the regression model (Figure 2B), and the AUC was 0.868 (95% CI: 0.815–0.920). When a predictive probability =0.113 was used as the cut-off value, the sensitivity and specificity of the nomogram for predicting RLN LN metastasis were 94.3% and 61.6%, respectively. The nomogram (Figure 4) that was constructed according to the regression model included the following three variables: short-axis diameter of the right RLN LN, tumour location and SLN status.
Analysis of left RLN LN metastasis alone
Multivariate analysis of left RLN LN metastasis alone
Univariate analysis (Table 1) showed that risk factors for left RLN LN metastasis included the short-axis diameter of the left RLN LN, maximum tumour diameter, SLN status and PLN status. Multivariate logistic regression analysis (Table 4) showed that the short-axis diameter of the left RLN LN was an independent risk factor for left RLN LN metastasis alone. We did not construct a nomogram, as there was only one independent risk factor from the available data.
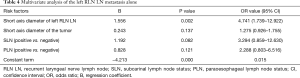
Full table
Evaluation of the fit of the regression model for left RLN LN metastasis alone
The Hosmer-Lemeshow test calculated that χ2=6.273, P=0.617. The AUC value calculated from the left RLN LN metastasis alone (Figure 2C) was 0.790 (95% CI: 0.688–0.892). When a predictive probability =0.036 was used as the cut-off value, the sensitivity and specificity of the logistic model for predicting left RLN LN metastasis alone were 95.2% and 31.1%, respectively.
Discussion
Locoregional LN recurrence is the main factor affecting the prognosis of ESCC patients postoperatively (4,16). LNs near the bilateral RLN are one of the most common locations of local recurrence. More importantly, RLN LN metastasis has been shown to be a strong predictor of poor prognosis in thoracic ESCC patients (17-19). Tabira et al. (18) reported that the 5-year survival rate of patients with RLN LN metastasis was 21%, which was significantly lower than that of patients with no RLN LN metastasis (47%). Some experts have considered that regular RLN LN dissection is necessary during surgery for ESCC because this process led to more precise staging and a reduction in the recurrence rate (20). Furthermore, RLN LN metastasis is an important risk factor for cervical LN metastasis (18-20). Therefore, comprehensive research should be conducted on RLN LN metastasis.
The RLN LN is one of the most common metastatic sites in oesophageal cancer, and previous research has reported that the RLN LN metastasis rate is 15–42% (19,21-23). Ye et al. (17) reported that the rates of left and right RLN LN metastasis were 15.8% and 20.8%, respectively, and Kanemura et al. (19) reported rates of 15.4% and 16.9%, respectively. Our research showed that the rates of left and right RLN LN metastasis were 15.10% and 20.40%, respectively, and the total metastatic rate of the bilateral RLN LNs was 28.3%, which was similar the results of a previous study. However, dissection of the RLN LNs causes the following problems. First, the procedure requires a trans-right thoracic resection of the oesophagus and lymphadenectomy and neck anastomosis, which leads to additional trauma. In addition, dissection of the LNs along the RLNs is technically challenging for thoracic surgeons. Above all, extensive lymphadenectomy along the RLNs leads to increased complications, such as RLN injury, pulmonary infection and anastomotic fistula (12,23-25), and leads to a poor quality of life (21). Taniyama et al. (11) showed that 36% of patients experienced RLN injuries, of which 16% were bilateral. Ma et al. (24) reported that RLN palsy occurred in 37.6% of patients. In the present study, the incidence of RLN injury was 13.6%, of which 53.8% (21/39) of cases led to subsequent pulmonary infection, which was a higher rate than that observed in the group without RLN injuries (7.9%, 18/229). Hence, surgical complications may be reduced for ESCC patients if unnecessary LN dissections are avoided.
Very few studies have reported the risk factors and pattern of RLN LN metastasis (24,26). Imaging experts have used FDG-PET/CT or intravenous contrast-enhanced CT to evaluate the risk of RLN LN metastasis; however, these modalities only showed limited predictive value (19,27,28). Yu et al. (26) made a rough estimate of the probability of RLN LN metastasis by dividing the risks into three grades, but the values used to determine the risk factors could not be obtained preoperatively.
Our statistical results showed that the short-axis diameter of the left RLN LN, the short-axis diameter of the right RLN LN, maximum tumour diameter of the, tumour location, SLN status and PLN status were all independent risk factors for RLN LN metastasis. In this study, we did not include T-stage in the multivariate analysis because it was unable to be determined preoperatively and had a strong linear correlation to the maximum tumour diameter. Endoscopic ultrasonography provides much information, but the accuracy is not satisfactory currently. Therefore, we did not include T-stage in our model in consideration of the clinical practicability. The data also showed that the rates of RLN LN metastasis for cases with SLN metastasis or not were 61.76% and 23.38%, respectively. Therefore, SLN status was a vital prognostic factor for RLN LN metastasis.
Finally, we formulated a logistic regression model and constructed a nomogram accordingly. The values of the abovementioned variables can be determined preoperatively or intraoperatively. For example, one patient with the lower third thoracic ESCC (0 points) underwent oesophagectomy, and the maximum diameters of the left (0 points) and right (0 points) RLN LNs were all less than 5 mm on CT. The maximum diameter of the tumour measured intraoperatively was 4 cm (40 points), and the intraoperative pathological analysis showed that the SLN status (0 points) and PLN status (0 points) were both negative. These six values summed to 40 points. Therefore, we determined that the risk of RLN LN metastasis was less than 0.1. As shown above, the risk of RLN LN metastasis can be determined according to the scoring system. The AUC of the model was 0.809, which proved that this scoring system had a high accuracy for predicting RLN LN metastasis.
However, our study had several limitations and shortcomings. First, our study design was retrospective, and the sample size was small; thus, selection bias was unavoidable, and further multi-institutional studies with larger sample sizes need to be performed in the future. Another limitation was the absence of clinical outcomes because of the short follow-up period. We have begun to collect prognostic information regarding RLN LN metastasis.
In summary, our study showed that upper thoracic ESCC patients with larger tumours and larger RLN LNs are more prone to RLN LN metastasis. Metastasis of the subcarinal and PLNs is also closely related to RLN LN metastasis. By quantifying the risk factors described above, we formulated a scoring system to accurately estimate the risk of RLN LN metastasis. However, the accumulation of more data and prospective studies are needed in the future to further improve the model and to provide more accurate guidance for clinical treatment.
Acknowledgments
We thank Dr. Ning Jiang for his help in collecting data (Department of Thoracic Surgery, The Second Hospital of Shandong University, Jinan, China) and Xiao Qiang and Yuan Zhang (Evidence-based Medicine Centre, The Second Hospital of Shandong University, Jinan, China) for their contributions to the statistical analysis in this article.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the Ethics Committee of the General Hospital of the Jinan Military Area (JN2018012) and the Second Affiliated Hospital of Shandong University (SD20180131). All patients provided written informed consent for their procedures.
References
- Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet 2013;381:400-12. [Crossref] [PubMed]
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. [Crossref] [PubMed]
- Tachibana M, Kinugasa S, Yoshimura H, et al. Clinical outcomes of extended esophagectomy with three-field lymph node dissection for esophageal squamous cell carcinoma. Am J Surg 2005;189:98-109. [Crossref] [PubMed]
- Nakagawa S, Kanda T, Kosugi S, et al. Recurrence pattern of squamous cell carcinoma of the thoracic esophagus after extended radical esophagectomy with three-field lymphadenectomy. J Am Coll Surg 2004;198:205-11. [Crossref] [PubMed]
- Ma GW, Situ DR, Ma QL, et al. Three-field vs two-field lymph node dissection for esophageal cancer: a meta-analysis. World J Gastroenterol 2014;20:18022-30. [Crossref] [PubMed]
- Altorki N, Kent M, Ferrara C, Port J. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177-83. [Crossref] [PubMed]
- Fujita H, Sueyoshi S, Tanaka T, et al. Optimal lymphadenectomy for squamous cell carcinoma in the thoracic esophagus: comparing the short- and long-term outcome among the four types of lymphadenectomy. World J Surg 2003;27:571-9. [Crossref] [PubMed]
- Mariette C, Piessen G. Oesophageal cancer: how radical should surgery be? Eur J Surg Oncol 2012;38:210-3. [Crossref] [PubMed]
- Shao L, Ye T, Ma L, et al. Three-field versus two-field lymph node dissection for thoracic esophageal squamous cell carcinoma: a propensity score-matched comparison. J Thorac Dis 2018;10:2924-32. [Crossref] [PubMed]
- Law S, Wong J. Two-field dissection is enough for esophageal cancer. Dis Esophagus 2001;14:98-103. [Crossref] [PubMed]
- Taniyama Y, Miyata G, Kamei T, et al. Complications following recurrent laryngeal nerve lymph node dissection in oesophageal cancer surgery. Interact Cardiovasc Thorac Surg 2015;20:41-6. [Crossref] [PubMed]
- Gockel I, Kneist W, Keilmann A, et al. Recurrent laryngeal nerve paralysis (RLNP) following esophagectomy for carcinoma. Eur J Surg Oncol 2005;31:277-81. [Crossref] [PubMed]
- Japan Esophageal Society. Japanese Classification of Esophageal Cancer, 11th Edition: part II and III. Esophagus 2017;14:37-65.
- Peters TT, Castelijns JA, Ljumanovic R, et al. Diagnostic value of CT and MRI in the detection of paratracheal lymph node metastasis. Oral Oncol 2012;48:450-5. [Crossref] [PubMed]
- Mizowaki T, Nishimura Y, Shimada Y, et al. Optimal size criteria of malignant lymph nodes in the treatment planning of radiotherapy for esophageal cancer: evaluation by computed tomography and magnetic resonance imaging. Int J Radiat Oncol Biol Phys 1996;36:1091-8. [Crossref] [PubMed]
- Li CL, Zhang FL, Wang YD, et al. Characteristics of recurrence after radical esophagectomy with two-field lymph node dissection for thoracic esophageal cancer. Oncol Lett 2013;5:355-9. [Crossref] [PubMed]
- Ye K, Xu JH, Sun YF, et al. Characteristics and clinical significance of lymph node metastases near the recurrent laryngeal nerve from thoracic esophageal carcinoma. Genet Mol Res 2014;13:6411-9. [Crossref] [PubMed]
- Tabira Y, Yasunaga M, Tanaka M, et al. Recurrent nerve nodal involvement is associated with cervical nodal metastasis in thoracic esophageal carcinoma. J Am Coll Surg 2000;191:232-7. [Crossref] [PubMed]
- Kanemura T, Makino T, Miyazaki Y, et al. Distribution patterns of metastases in recurrent laryngeal nerve lymph nodes in patients with squamous cell esophageal cancer. Dis Esophagus 2017;30:1-7. [PubMed]
- Li H, Yang S, Zhang Y, et al. Thoracic recurrent laryngeal lymph node metastases predict cervical node metastases and benefit from three-field dissection in selected patients with thoracic esophageal squamous cell carcinoma. J Surg Oncol 2012;105:548-52. [Crossref] [PubMed]
- Baba M, Aikou T, Natsugoe S, et al. Quality of life following esophagectomy with three-field lymphadenectomy for carcinoma, focusing on its relationship to vocal cord palsy. Dis Esophagus 2017;11:28-34. [Crossref] [PubMed]
- Wu J, Chen QX, Zhou XM, et al. Does recurrent laryngeal nerve lymph node metastasis really affect the prognosis in node-positive patients with squamous cell carcinoma of the middle thoracic esophagus? BMC Surg 2014;14:43. [Crossref] [PubMed]
- Sato Y, Kosugi S, Aizawa N, et al. Risk Factors and Clinical Outcomes of Recurrent Laryngeal Nerve Paralysis After Esophagectomy for Thoracic Esophageal Carcinoma. World J Surg 2016;40:129-36. [Crossref] [PubMed]
- Ma L, Xiang J, Zhang Y, et al. Characteristics and clinical significance of recurrent laryngeal nerve lymph node metastasis in esophageal squamous cell carcinoma. J BUON 2017;22:1533-9. [PubMed]
- Koyanagi K, Igaki H, Iwabu J, et al. Recurrent Laryngeal Nerve Paralysis after Esophagectomy: Respiratory Complications and Role of Nerve Reconstruction. Tohoku J Exp Med 2015;237:1-8. [Crossref] [PubMed]
- Yu S, Lin J, Chen C, et al. Recurrent laryngeal nerve lymph node dissection may not be suitable for all early stage esophageal squamous cell carcinoma patients: an 8-year experience. J Thorac Dis 2016;8:2803-12. [Crossref] [PubMed]
- Kato H, Kuwano H, Nakajima M, et al. Comparison between positron emission tomography and computed tomography in the use of the assessment of esophageal carcinoma. Cancer 2002;94:921-8. [Crossref] [PubMed]
- Billè A, Okiror L, Skanjeti A, et al. Evaluation of integrated positron emission tomography and computed tomography accuracy in detecting lymph node metastasis in patients with adenocarcinoma vs squamous cell carcinoma. Eur J Cardiothorac Surg 2013;43:574-9. [Crossref] [PubMed]


