Uniportal video-assisted sleeve resections: how to deal with specific challenges
Introduction
Concerns about incomplete resection and future recurrence, combined with the fact that a parenchyma sparing procedure is more technically demanding than that of a pneumonectomy, have traditionally reserved sleeve pulmonary resection (SL) procedures for patients diagnosed with central lung tumors who cannot tolerate a pneumonectomy. However, in the light of evidence suggesting better outcomes after SL, the former, are now officially recommended for the treatment of central lung tumors involving the orifice of the lobar bronchus or extending into the main bronchus and in case the hilar lymph nodes are infiltrated, irrespective of the patient’s capacity to tolerate a pneumonectomy (1,2). Quite recently, Pages et al. published a retrospective study of 6,259 patients registered at the French database, of whom, 941 were submitted to sleeve lobectomy and 5,318 to pneumonectomy for non-small cell lung cancer (NSCLC) between 2005–2014. The authors concluded that the 3-year overall survival (OS) and disease-free survival (DFS) were higher in the SL compared to the pneumonectomy group. SL also demonstrated favorable outcomes regarding the incidence of bronchopleural fistula, empyema occurrence and postoperative arrhythmia, but not regarding atelectasis and pneumonia (3). Shortly after, Abdelsattar et al. reviewed data from 23,964 patients (1,713 underwent a SL, while 22,251 a pneumonectomy), registered at the United States’ National Cancer Database (NCDB) operated on from 1998 to 2012 for NSCLC and carcinoid. Their findings confirmed the results of the French group regarding improved OS following SL compared to pneumonectomy. Moreover, pneumonectomy was associated with higher 30 and 90-day mortality rate compared to SL (4). Preserved pulmonary reserves, the avoidance of potentially fatal complications, such as bronchopleural fistula and postpneumonectomy pulmonary oedema, the preservation of right ventricular function which in case of a pneumonectomy could deteriorate due to increased resistance of the pulmonary vasculature, and the option to treat a recurrence amenable to surgical excision, should it occur, plus improved quality of life (QoL) and cost effectiveness, also account for the increasing implementation of SL in cases where it is technically and oncologically feasible (5,6). Preservation of the parenchyma may sometimes call for a combined bronchial and vascular sleeve resection (bronchovascular or double sleeve resection). As with bronchial, bronchovascular sleeves have also favorable outcomes compared to pneumonectomy (7).
Technically demanding sleeve resections may be, however advances in imaging systems and surgical instruments design, the accumulation of experience in video-assisted thoracic surgery (VATS) for standard lobectomy, as well as the well documented advantage of VATS over open surgery for major lung resections in terms of postoperative pain and morbidity, length of hospital stay, postoperative QoL, adjuvant therapy tolerance and probably long term survival, have prompted surgeons to adopt minimally invasive techniques for cases requiring a sleeve resection (8-13). Thus, the benefits of a parenchyma sparing procedure are combined with those of VATS. Since the first report in 2002 of a bronchial sleeve resection, case reports, a few small and one large case series study as well as a retrospective comparative study, have provided robust evidence that not only VATS sleeve resections are feasible, but also yield results comparable to those yielded by open surgery (14-16). Almost all the patients presented in these case series were operated on via a multiport technique. The first report of a uniportal bronchial sleeve lobectomy in 2013, followed by numerous others, including small size (<10) case series studies, demonstrated the safety, and feasibility of the single-port technique in the context of sleeve bronchovascular resections (17,18). A recent single center, retrospective study on the outcomes of 79 consecutive uniportal VATS bronchial sleeve lobectomies, demonstrated favorable 1- and 2-years OS, 30-day mortality and morbidity for patients who were offered a uniportal VATS bronchial sleeve lobectomy for the treatment of centrally located pulmonary tumors, providing robust evidence not only on the feasibility and safety, but also on the oncologic efficacy of the technique (19).
The uniportal VATS technique presents certain differences compared to multiport techniques, attributed to the fact that the instruments and the camera are all placed through a single 3–4 cm incision. Apart from the need for bronchial and vascular resection and reconstruction, cases requiring a sleeve resection, also present certain common characteristics, such as the central location and the size of the culprit lesion, the potentially inaccessible hilum due to enlarged or infiltrated lymph nodes or fibrosis due to the underlying inflammatory process attributed to the mass effect of the lesion or previously administered induction therapy, and the retention of air due to obstruction of the lobar orifice, which pose difficulties, usually not present during a standard resection. Herein we attempt to introduce a standardized technical approach to counter certain challenges encountered when dealing with cases requiring a bronchial or bronchovascular sleeve resection via a single incision access, in order to simplify the decision making process regarding the optimal intraoperative strategy.
Preoperative planning
Staging is conducted as recommended in already published guidelines (1,2). A millimetric-thin sliced contrast enhanced computed tomography (CT) of the chest is of great value when planning the operative strategy for a sleeve resection. Study of all three dimensions (axial, coronal, sagittal) provide a clear understanding of the lesion’s location and relation with the hilar bronchovascular structures, azygos arch, superior vena cava (right side) and aortic arch (left side), as well as of the extent of their adherence or infiltration. Magnetic resonance imaging (MRI) is useful when vascular invasion is suspected. A 3D reconstruction or even 3D-printing may further facilitate the preoperative planning, although the latter is still not routinely applied (20). The bronchial tree should also be assessed bronchoscopically in order to identify the intraluminal extension of the malignancy and clarify the proximal and distal site of the transection (21).
Technique
Site of incision
Technically, the successful completion of a sleeve resection is mainly determined by the integrity and the sufficient caliber of the reconstruction. Thus, the site of the incision is of paramount importance in uniportal VATS, as it can facilitate or impair the efficiency of the suturing. Siting the incision at the 4th intercostal space, anterior axillary line, provides a direct access to both ends of the bronchial tree and/or artery and enables suturing in an axis almost perpendicular to that of the anastomosis, just like in open surgery (22). Rotating the table anteriorly or posteriorly may improve the exposure when suturing the far or near edge of the anastomosis respectively.
Reconstruction
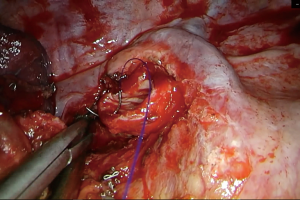
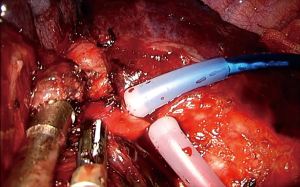
For the reconstruction of the bronchial tree a number of suture materials and techniques have been described, all having proven in practice equally reliable and effective (18,23-25). Interrupted, or continuous, absorbable or non, series of both open and VATS (uni and multi-port) sleeve lobectomies have demonstrated that any suturing technique can be utilized for the bronchial reconstruction, provided that the anastomosis is tension free and adequately vascularized (23-25). Regarding the blood supply of the anastomosis, it should be noted that the transection of the bronchial artery is compensated by the rich anastomotic network between the pulmonary and bronchial arterial circulation, which, at the level of the lobar bronchus, accounts for as much as 75–90% of the bronchial blood supply (26). A consensus seems to exist regarding the inappropriateness of silk sutures as they result in granulation with subsequent stenosis of the anastomosis (27). Currently, we use a continuous, monofilament, non-absorbable, 3/0 double-needle suture (Figure 1). Such a suture has been claimed to reduce the rotational deformity of the anastomosis observed bronchoscopically over the years (28). However, the main reason for our preference is that a continuous suture can reduce the time required for the completion of the anastomosis, not only because additional sutures are not required, but also because tangling is minimized when using a single suture. The first bite is placed “inside-out” at the far border between the cartilaginous and membranous part of the proximal bronchial tree and connected accordingly “outside-in” distally, incorporating the entire bronchial wall (full-thickness). Suturing is continued with the same needle in an anticlockwise direction, along the medial side of the circumference of the anastomosis, until half of it has been completed. During the suturing, appropriate tension is exercised by the assistant to ensure firm reapproximation of the proximal and distal bronchial tree and prevent the suture form loosening. Suturing is then resumed with the second needle, starting “inside-out” distally (at the far end of the anastomosis) and connecting accordingly, “outside-in”, at the proximal bronchial tree and progressing in a clockwise direction, along the lateral side, towards the near end of the anastomosis until the first suture is met. The suture is held relatively loose for the last 2–3 bites, in order to maintain direct vision of the proximal and distal lumen, ensuring correct placement of the needle. Before tying, tension should be equally distributed along the lateral side of the anastomosis, using, if needed, a hook. The distance between the bites is usually 2 mm. However, if there is a discrepancy between the caliber of the proximal and distal bronchus, such as when suturing the middle lobe to the proximal bronchus intermedius after a right lower sleeve lobectomy, or the right upper lobe bronchus to the carina following a tracheal sleeve bilobectomy, the distance between the bites should be adjusted accordingly. In particular, the bites should be placed relatively sparsely at the proximal tree and densely distally. Discrepancy is usually not a problem when suturing the right upper lobe bronchus to the right main, and the left upper or lower to the left main bronchus. When anastomosing the left lower to the left main bronchus following a left upper sleeve lobectomy, suturing starts from the lower lobe bronchus and connects accordingly to the left main (distal to proximal and not proximal to distal as previously described).
The reconstruction of the bronchial tree is facilitated when dissection of lymph node stations 2R, 4R (right side), 4L (left side) and 7 is carried out prior to the anastomosis.
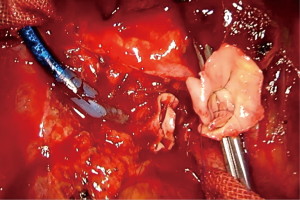
For a vascular reconstruction following a vascular sleeve resection, the suturing technique we apply is identical as the one described above. A 4/0 or 5/0 double needle (no more than 16 mm) prolene suture is preferred (Figure 2). It is recommended to irrigate both stumps of the artery with a syringe, before starting the reconstruction, in order to wash away debris from the endothelium and prevent subsequent pulmonary embolism.
In case of a bronchovascular sleeve resection, transection of the artery precedes that of the bronchus, while reconstruction of the bronchus precedes that of the artery.
After the completion of a bronchovascular reconstruction, it is advisable to keep the suture lines of the arterial and bronchial anastomosis from contacting each other, in order to prevent the formation of an arterial-bronchial fistula, by interposing a strip of mediastinal pleura (29).
It should be noted that whenever a sleeve (bronchial, vascular or bronchovascular) lobectomy is performed, a frozen section biopsy of the excision margins should be obtained prior to the anastomosis. If the excision margin is found to be infiltrated by cancerous cells, an additional portion of the structure (bronchus or artery) should be resected, until the margins are clear.
Tension
Releasing the inferior pulmonary ligament, usually suffices to minimize the tension of the anastomosis. If tension is still an issue, a “U” shaped incision of the pericardium (intrapericardial release) just inferior to the lower pulmonary vein, extending anterior (posterior to the phrenic nerve) and posterior to it provides a solution (30).
Blood supply of the bronchial anastomosis
Regarding the blood supply of the anastomosis, extensive devascularization may lead to ischemia, inadequate healing and anastomotic related complications (dehiscence, fistula, stenosis), thus skeletonization of the bronchial tree should be avoided, although not to the expense of lymph node dissection (31).
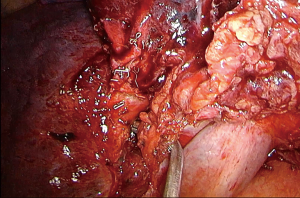
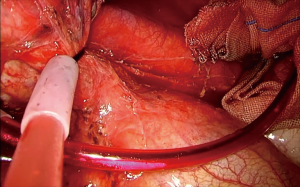
Pericardial incision: azygos arch division (Figure 3)

These maneuvers can prove very useful for a right and left upper lobe sleeve resection, and upper sleeve bilobectomy. The right upper lobe is the most commonly performed sleeve resection. It is considered as the less demanding, due to the fact that the right main bronchus is by default aligned with the bronchus intermedius, which greatly facilitates the reconstruction. The superior pulmonary vein is usually transected first, followed by the truncus anterior and any separate arterial branches for the anterior or posterior segment, arising from the main pulmonary artery (PA). The transverse and posterior part of the oblique fissure are completed, the inferior pulmonary ligament released, lymph node stations 7, 2R and 4R dissected and the bronchial tree incised proximally at the main bronchus and distally at the bronchus intermedius as proximally as possible, ensuring free margins.
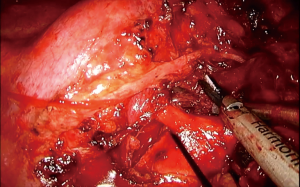
This order of dissection is convenient, provided the hilum is accessible. However, bulky tumors, enlarged hilar lymph nodes, fibrosis due to the inflammatory process triggered via a mass effect reaction or because of previous induction therapy, or even direct invasion from the tumor itself, may deem the hilum inaccessible as far as the azygos—SVC junction. The mobilization of the lobe in such a scenario can be very limited, as well as the access to the fissure, especially if it is incomplete. Incising the pericardium provides a way out. The phrenic nerve should be transected if it is involved. The incision can extend in a cephalad direction up to the insertion of the SVC and caudally at the level of the cavo-atrial junction. The superior pulmonary vein can now be stapled, opening up the plane for the dissection of the truncus anterior (Figure 4). A wide pericardial incision can facilitate the transection of the truncus anterior, even before that of the superior pulmonary vein, as it significantly enhances the maneuverability of the lobe. The access to the hilum can be further augmented, if needed, with division of the azygos arch at the level of the azygos-caval junction. In case the superior vena cava is infiltrated and the tumor can be adequately removed with a partial resection, then division of the azygos arch is mandatory (Figure 5). If both opening the pericardium and dividing the arch are necessary, the sequence of implementation is decided by the surgeon depending on the case.

Similarly, in a left upper sleeve resection, opening the pericardium (posterior to the phrenic nerve) can provide the space needed to transect the left superior pulmonary vein in case the hilum is inaccessible, and the fissure fused. Also, it helps obtain a very proximal arterial control, if needed, together with the transection of the arterial ligament. After transecting the vein, the plane is freed for the transection of the arterial branches, the dissection of the fissure and the bronchi.
Following the completion of the surgery, the pericardium needs not to be reconstructed, as the risk of cardiac herniation in resections other than pneumonectomy is very low (34).
Pulmonary artery and inferior pulmonary vein clamping (Figure 6)

Cases requiring occlusion of the pulmonary artery are usually tumors of the upper lobes. The tumor may be directly invading the main arterial trunk or encapsulating one or more arterial branches up to their orifice making their ligation and transection difficult. In the first situation a proper circumferential arterial section proximally and distally is needed, followed by an end-to-end anastomosis (vascular sleeve). In the second situation, the involved tributary(ies) can be transected at its orifice with scissors and the main trunk sutured after the removal of the lobe (plasty). Again, proximal and distal occlusion is necessary. Clamping the PA, of course, can be utilized in any other situation when proximal vascular control is required for safety reasons. In the left side, when very proximal control is required, the arterial ligament can be transected (Figure 7).
This maneuver can also be utilized to obtain better exposure for the reconstruction of an anastomosis after a bronchial sleeve resection. Exposure of the proximal and distal bronchus for reconstructions following middle, right upper (sometimes) and left upper (often) bronchial sleeve resections is significantly improved when the PA is snared, and this may favorably impact on the time required to complete the anastomosis.
The PA can be clamped either with a vascular clamp or snared with a tourniquet. A tourniquet is more convenient, as it can be placed into the thorax, thus not occupying the incision and limiting the movements of the surgeon. When using a tourniquet for distal arterial control during a left bronchovascular resection, it is advisable to separately snare the superior segmental artery (A6) from the basal arterial trunk (Figure 8). Removing lymph node stations 10, 5, and 4L, in the left side, and #11 and 4R in the right side facilitates the snaring of the PA and later on the reconstruction.
Intravenous heparin administration before clamping can prevent thrombosis and subsequent pulmonary embolism. Doses ranging from 1,500–5,000 IU have been reported (36).
When during an upper lobe resection requiring a vascular sleeve, distal arterial control cannot be achieved, then the inferior pulmonary vein can be snared instead (Figure 9). After the transection of the superior pulmonary vein, the PA can be sectioned proximally and then the bronchus, followed by the distal PA. Although this maneuver can suffice in preventing the backflow coming from the distal PA, still some bleeding may exist, attributed to collateral network between the bronchial arterial circulation and the pulmonary arterial circulation.
After the reconstruction, before tying, gradually resume the circulation by removing the distal clamp first, in order to de-air the artery and prevent air embolism.
Conclusions
VATS bronchial and bronchovascular sleeve resections are not only feasible, but also add the advantages of minimally invasive surgery to those of parenchyma sparing procedures. In the same time, the technical complexity increases, especially when the operation is conducted via a single 3–4 cm incision. Cases calling for a sleeve resection, quite often share certain common characteristics such as the tumor’s central location and size and enlarged lymph nodes, which are less often encountered during a standard resection. As a result, the surgeon is likely to face problems such as inaccessible hilum, fused fissure, inflated lung, involvement of the PA, which result in impaired maneuverability of the lung and limited access to surgical planes. A “road map” consisting of certain maneuvers, readily applicable by the surgeon, could not only reduce the duration, but, most importantly, ensure the safety of the procedure. This is particularly true for teams who are going through their learning curve regarding uniportal sleeve resections.
Such a road map could consist from the following points: (I) whenever access to the hilum is blocked and proceeding through the fissure is not an option, consider incising the pericardium. This will provide the space required to deal with the superior vein (right or left) and then proceed with the arterial branches and the fissure. In the left side, opening the pericardium will also facilitate proximal vascular control. Repair of the pericardium, following procedures other than pneumonectomy is not necessary. (II) In the right side, if the access still remains limited, or if direct invasion of the tumor calls for it, transect the azygos arch, at the level of the azygos—SVC junction. (III) Whenever vascular control is necessary, even if not a vascular sleeve is needed, clamp or ensnare the PA proximally and distally. Proximal vascular control in the left side is facilitated by removing lymph nodes #10, 5, 4L and in the right, #11, 4R. Ensnaring the artery is preferred over clamping, because the tourniquets can be placed into the chest, sparing the space of the single incision for the surgeon and the camera. Distal vascular control in the left side, is better attained by ensnaring the superior and basal arterial branches separately. (IV) Transect the arterial ligament if very proximal arterial control is needed in the left side. (V) Clamp the inferior pulmonary vein whenever distal arterial control is difficult. (VI) Release the inferior pulmonary ligament to ensure a tension free anastomosis. (VII) Perform an intrapericardial release, if tension is still an issue. (VIII) Following the completion of a bronchovascular reconstruction, separate the suture line of the arterial and bronchial anastomosis to prevent the formation of a fistula.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
References
- Detterbeck F, Zelman Lewis S, Diekemper R, et al. Diagnosis and Management of Lung Cancer, 3rd ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2013;143:7S-37S.
- National Comprehensive Cancer Network. Non-Small Cell Lung Cancer (Version 2.2019). Available online: https://www.nccn.org/professionals/physician_gls/pdf/nscl_blocks.pdf
- Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-195.e3. [Crossref] [PubMed]
- Abdelsattar ZM, Shen R, Yendamuri S, et al. Outcomes After Sleeve Lung Resections Versus Pneumonectomy in the United States. Ann Thorac Surg 2017;104:1656-64. [Crossref] [PubMed]
- Martin-Ucar AE, Chaudhuri N, Edwards JG. Can pneumonectomy of non-small cell lung cancer be avoided? An audit of parenchymal sparing lung surgery. Eur J Cardiothorac Surg 2002;21:601-5. [Crossref] [PubMed]
- Ferguson MK, Lehman AG. Sleeve lobectomy or pneumonectomy: optimal management strategy using decision analysis techniques. Ann Thorac Surg 2003;76:1782-8. [Crossref] [PubMed]
- Lausberg HF, Graeter T, Tscholl D, et al. Bronchovascular Versus Bronchial Sleeve Resection for Central Lung Tumors. Ann Thorac Surg 2005;79:1147-52. [Crossref] [PubMed]
- Bendixen M, Jørgensen OD, Kronborg C. Postoperative pain and quality of life after lobectomy via video-assisted thoracoscopic surgery or anterolateral thoracotomy for early stage lung cancer: a randomised controlled trial. Lancet Oncol 2016;17:836-44. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg. 2010;139:366-78. [Crossref] [PubMed]
- Handy JR Jr, Asaph JW, Douville EC, et al. Does video assisted thoracoscopic lobectomy for lung cancer provide improved functional outcomes compared with open lobectomy? Eur J Cardiothorac Surg 2010;37:451-5. [PubMed]
- Zhang Z, Zhang Y, Feng H. Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg 2013;44:407-14. [Crossref] [PubMed]
- Petersen RP, Pham D, Burfeind W., et al. Thoracoscopic Lobectomy Facilitates the Delivery of Chemotherapy after Resection for Lung Cancer. Ann Thorac Surg 2007;83:1245-9. [Crossref] [PubMed]
- Taioli E, Lee DS, Lesser M, Flores R. Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg 2013;44:591-7. [Crossref] [PubMed]
- Santambrogio L, Cioffi U, De Simone M, et al. Video-assisted sleeve lobectomy for mucoepidermoid carcinoma of the left lower lobar bronchus: a case report. Chest 2002;121:635-6. [Crossref] [PubMed]
- Zhou S, Pei G, Han Y. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg 2015;10:116. [Crossref] [PubMed]
- Huang J, Li J, Qiu Y, et al. Thoracoscopic double sleeve lobectomy in 13 patients: a series report from multi-centers. J Thorac Dis 2015;7:834-42. [PubMed]
- Gonzalez-Rivas D, Fernandez R, Fieira E, et al. Uniportal video-assisted thoracoscopic bronchial sleeve lobectomy: first report. J Thorac Cardiovasc Surg 2013;145:1676-7. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Uniportal Video Assisted Thoracoscopic sleeve lobectomy and bronchoplasty: initial experience Interact Cardiovasc Thorac Surg 2014;17:601-2.
- Soultanis KM, Chen Chao M, Chen J, et al. Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 2019. [Epub ahead of print]. [Crossref] [PubMed]
- Giannopoulos AA, Steigner ML, George E, et al. Cardiothoracic Applications of 3-dimensional Printing. J Thorac Imaging 2016;31:253-72. [Crossref] [PubMed]
- Predina JD, Kunkala M, Aliperti L., et al. Sleeve Lobectomy: Current Indications and Future. Directions. Ann Thorac Cardiovasc Surg 2010;16:310-8. [PubMed]
- Gonzalez-Rivas D, Yang Y, Stupnik T, et al. Uniportal video-assisted thoracoscopic bronchovascular, tracheal and carinal sleeve resections. Eur J Cardiothorac Surg 2016;49:i6-16. [PubMed]
- Merritt RE, Mathisen D, Wain J, et al. Long-Term Results of Sleeve Lobectomy in the Management of Non-Small Cell Lung Carcinoma and Low-Grade Neoplasms. Ann Thorac Surg 2009;88:1574-81. [Crossref] [PubMed]
- Huang J, Li S, Hao Z, et al. Complete video-assisted thoracoscopic surgery (VATS) bronchial sleeve lobectomy. J Thorac Dis 2016;8:553-74. [Crossref] [PubMed]
- Agasthian T. Initial experience with video-assisted thoracoscopic bronchoplasty Eur J Cardiothorac Surg 2013;44:616-23. [Crossref] [PubMed]
- Fréchette E, Deslauriers J. Surgical Anatomy of the Bronchial Tree and Pulmonary Artery. Semin Thorac Cardiovasc Surg 2006;18:77-84. [Crossref] [PubMed]
- Frist WH, Mathisen DJ, Hilgenberg AD. Bronchial sleeve resection with and without pulmonary resection. J Thorac Cardiovasc Surg 1987;93:350-7. [PubMed]
- Mentzer S. Right Upper Lobe Sleeve Resection. Oper Tech Thorac Cardiovasc Surg 1998;3:166-77. [Crossref]
- Abe J, Hasumi T, Takahashi S, et al. Fatal broncho-pulmonary artery fistula after lobectomy for lung cancer†. J Surg Case Rep 2015;2015. [Crossref] [PubMed]
- Broussard B, Mathisen DJ. Tracheal release maneuvers. Ann Cardiothorac Surg 2018;7:293-8. [Crossref] [PubMed]
- Faber LP, Jensik R, Kittle F. Results of Sleeve Lobectomy for Bronchogenic Carcinoma in 101 Patients. Ann Thorac Surg 1984;37:279-85. [Crossref] [PubMed]
- Soultanis KM, Gonzalez-Rivas D. Intrapericardial upper sleeve bilobectomy with division of the azygos arch. Asvide 2019;6:221. Available online: http://www.asvide.com/watch/32906
- Soultanis KM, Gonzalez-Rivas D. Intrapericardial upper bilobectomy with SVC partial resection due to invasion by the tumor. Asvide 2019;6:222. Available online: http://www.asvide.com/watch/32907
- Parshad S, Lohchab S, Karwasra R, et al. Cardiac herniation following right lower lobectomy—a rare clinical entity. Indian J Thorac Cardiovasc Surg 2017;33:244-6. [Crossref]
- Soultanis KM, Gonzalez-Rivas D. Left upper lobe double sleeve lobectomy. Asvide 2019;6:223. Available online: http://www.asvide.com/watch/32908
- Maurizi G, D’Andrilli A, Venuta F, et al. Bronchial and arterial sleeve resection for centrally-located lung cancers. J Thorac Dis 2016;8:S872-81. [Crossref] [PubMed]