How can CO2-derived indices guide resuscitation in critically ill patients?
Introduction
In patients with acute circulatory failure, one of the goals of the treatment is to increase cardiac output. The aim is to improve the oxygen delivery to the tissues and correct the mismatch between oxygen demand and supply, which is the hallmark of shock (1). However, no absolute normal value of cardiac output or oxygen delivery can be defined, as their adequate value basically depends on the tissue oxygen requirements. The correct value of cardiac output is the one that ensures a flow of oxygen that meets the metabolic demand (2,3). Then, any treatment aimed at changing cardiac output, such as fluid or inotropes, must be driven by the assessment of the adequacy between oxygen demand and supply.
To assess this adequacy, clinical examination has still a limited value. Signs of skin hypoperfusion do not reliably detect tissue hypoxia (4). Urine output may reflect the kidney perfusion, but it might be altered by many other factors during shock. Moreover, it depends on the presence or absence of a prior renal failure, and it cannot be used anymore as an indicator of the kidney perfusion in the case of acute tubular necrosis (5). Blood lactate may increase due to many processes not related to tissue oxygenation, leading to false positives (6). Furthermore, the blood lactate concentration depends on the balance between lactate production and lactate clearance, thus the delay required by its metabolism precludes one using it as a real-time marker of tissue metabolism (7). Oxygen saturation of the mixed (SvO2) or the central (ScvO2) venous blood is often in the normal range in septic shock despite anaerobic metabolism, because of the alteration of tissue oxygen extraction (8).
In this context, the indices derived from the arterial and central or mixed venous blood partial tension in carbon dioxide (CO2) were proposed to overcome many of the limitations of the previous variables to indicate the adequacy of oxygen supply and requirements (9).
The meaning of PCO2 gap
What is the PCO2 gap?
The difference between the mixed venous content (CvCO2) and the arterial content (CaCO2) of CO2 reflects the balance between its production by the tissues and its elimination through the lungs. This venoarterial difference in CO2 content (CCO2) can be estimated at the bedside by the venoarterial difference in PCO2 (PvCO2 − PaCO2), named PCO2 gap or ∆PCO2.
It is not possible to understand its clinical value without understanding how CO2 is produced, transported and eliminated, in aerobic and anaerobic conditions.
CO2 production
Under normoxic conditions, CO2 is produced in the cells during oxidative metabolism. The CO2 production (VCO2) is directly related to the global O2 consumption (VO2) by the relation:
VCO2 = R × VO2 [1]
where R is the respiratory quotient. R may vary from 0.7 to 1 depending on the predominant energetic substrate (0.7 for lipids, 1 for carbohydrates). Therefore, under aerobic conditions, CO2 production should increase either because the aerobic metabolism increases or, for a given VO2, because more carbohydrates are used as energetic substrates.
Under hypoxic conditions, CO2 is produced in the cells through buffering of excessively produced protons by local bicarbonate ions (HCO3−). Protons are generated by two mechanisms (10). First, CO2 increases because of the hydrolysis of adenosine triphosphate and of adenosine diphosphate that occurs in anaerobic conditions. Second, a potential but minor source of CO2 production under anaerobic conditions is the decarboxylation of some substrates produced by intermediate metabolism (α ketoglutarate or oxaloacetate) (10).
How is CO2 transported?
CO2 is transported in the blood in three forms: dissolved (10%), carried in bicarbonate ions (60%) and associated with proteins as carbamino compounds (30%). Compared to what happens for O2, the dissolved form of CO2 plays a more significant role in its transport because CO2 is approximately 20 to 30 times more soluble than O2. However, the main proportion of CO2 is carried in bicarbonates, which result from the reaction of CO2 and water molecules:
CO2 + H2O ↔ H2CO3 ↔ HCO3- + H+ [2]
From the tissues, CO2 diffuses into the red blood cells, where erythrocytic carbonic anhydrase catalyses CO2 hydration, converting most CO2 and H2O to HCO3− and H+ (11). In the red blood cells, dissolved CO2 can also be fixed by haemoglobin. This fixation depends on the oxidation state of haemoglobin, since CO2 has a greater affinity for reduced than for oxygenated haemoglobin (12). This is called the “Haldane effect” (13,14). In the peripheral capillaries this phenomenon facilitates the loading of CO2 by the blood, while O2 is delivered to the tissues. By contrast, in the lungs, the Haldane effect enhances the unloading of CO2 while O2 is transferred to haemoglobin.
Finally, the carbamino compounds are formed by combining the CO2 with the terminal NH2 groups of proteins, especially with the globin of haemoglobin. This reaction is also favoured by haemoglobin deoxygenation.
How is CO2 eliminated?
The three forms of CO2 are carried by the blood flow to pulmonary circulation and eliminated by ventilation. Passive diffusion from the capillaries to the alveoli eliminates CO2, depending on the difference in the gas tension between both spaces.
What is the relationship between CCO2 and PCO2?
Since CCO2 results from the combination of the three forms by which CO2 is transported, the formula to calculate it is complex and not practical for clinical purposes (15). In this regard, the possibility to derive CCO2 from one single component, notably the PCO2, is useful:
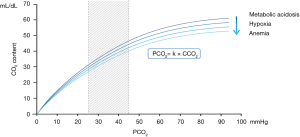
PCO2= k × CCO2 [3]
The k value is influenced by the degree of blood pH, haematocrit and the arterial oxygen saturation (16-18) (Figure 1). As a matter of fact, the relationship between CCO2 and PCO2 is almost linear over the physiological range (Figure 1). Then, in clinical practice, the PCO2 gap is an estimate of the difference between venous and arterial CO2 content (Cv-aCO2).
What are the determinants of the PCO2 gap?
According to the Fick equation applied to CO2, the CO2 excretion (which equals CO2 production—VCO2—in steady state) equals the product of cardiac output by the difference between mixed venous CCO2 (CvCO2) and arterial CCO2 (CaCO2):
VCO2 = cardiac output × (CvCO2 − CaCO2) [4]
As mentioned above, under physiological conditions, CCO2 can be substituted by PCO2 (PCO2= k × CCO2) so that:
∆PCO2 = k × (CvCO2 − CaCO2) [5]
and
VCO2 = cardiac output × ∆PCO2/k [6]
Thus, ∆PCO2 can be calculated from a modified Fick equation:
∆PCO2 = (k × VCO2)/cardiac output [7]
where k is the factor cited above in the relationship between PCO2 and CCO2.
This relationship between ∆PCO2 and cardiac output expresses the fact that, if cardiac output is low, the CO2 clearance decreases, CO2 stagnates at the venous side and PvCO2 increases relatively to PaCO2 at the venous level: this leads to an increase in the PCO2 gap.
In other words, for a given VCO2, a decrease in cardiac output results in an increased PCO2 gap and vice versa. This was found by experimental studies in which, when cardiac output was gradually reduced under conditions of stable VO2, the PCO2 gap was observed to concomitantly increase (9,19). Conversely, in a clinical study performed in normolactatemic patients with cardiac failure, the increase in cardiac index induced by dobutamine was associated with a decrease in the PCO2 gap, while VO2 was unchanged (20).
How to use the PCO2 gap in clinical practice?
Can ∆PCO2 be used as a marker of tissue hypoxia? No!
During cardiac arrest large increases in ∆PCO2 were reported suggesting that ∆PCO2 can increase during tissue hypoxia (21,22). However, because of the physiologic facts explained above, ∆PCO2 is not a straightforward indicator of anaerobic metabolism.
Indeed, in case of tissue hypoxia, ∆PCO2 can increase, decrease or remain unchanged, since the determinants of ∆PCO2 can change in opposite directions.
First, as mentioned above, the k factor (defining the relationship between PCO2 and CCO2) increases in case of tissue hypoxia, increasing the PCO2 gap even if the venoarterial difference in CCO2 does not change (artefactual increase of ∆PCO2).
Second, during tissue hypoxia, CO2 production should decrease as a result of the decrease in VO2: the less O2 is consumed, the less CO2 is produced. In an animal study where cardiac output was experimentally decreased by tamponade, Zhang and Vincent observed that, below a critical level of O2 delivery, the further decrease in both cardiac output and O2 delivery resulted in a progressive decrease in VCO2 along with the decrease in VO2 (9).
Since during tissue hypoxia, k must increase (tending to increase ∆PCO2) and VCO2 must decrease (tending to decrease ∆PCO2), the resultant effect on ∆PCO2 will mainly depend on cardiac output [∆PCO2 = (k × VCO2)/cardiac output] (23).
Therefore, two situations should be distinguished: tissue hypoxia with reduced blood flow and tissue hypoxia with preserved or high blood flow (Figure 2).
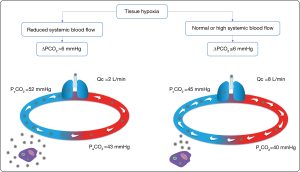
In cases of tissue hypoxia with reduced systemic blood flow, PvCO2 increases relatively to PaCO2 due to the venous stagnation phenomenon, which increases ∆PCO2. In this regard, in experimental studies where tissue hypoxia was induced by reducing blood flow, high values of ∆PCO2 were found (19,24).
On the other hand, in cases of tissue hypoxia with preserved or high systemic blood flow ΔPCO2 should be normal or even reduced. The high efferent venous blood flow should be sufficient to wash out the CO2 produced by the tissues, preventing stagnation and ∆PCO2 increase.
Results from several clinical studies have supported this hypothesis. Bakker et al. (25) found that most patients with septic shock had a ∆PCO2 ≤6 mmHg. Cardiac index obtained in this subgroup of patients was significantly higher than that obtained in the subgroup of patients with a ∆PCO2 >6 mmHg. Interestingly, the two subgroups did not differ in terms of blood lactate. Although VCO2 and VO2 were not directly measured, these data suggest that differences in CO2 production did not account for differences in ∆PCO2. In other words, many patients had a normal ∆PCO2 despite tissue hypoxia, probably because their high blood flow had easily removed CO2 produced by the tissues. Similar findings were reported by Mecher et al. (26). Clearly, these latter studies (25,26) underline the poor sensitivity of ∆PCO2 to detect tissue hypoxia.
Normal or low ∆PCO2 values were also reported in hypotensive patients with fulminant hepatic failure with tissue hypoxia, as strongly suggested by the increase in VO2 after prostacyclin infusion (27). At baseline ∆PCO2 was very low, which was probably due to the fact that VCO2 was low—as suggested by the low VO2—and that cardiac output was very high. These findings strongly support the fact that high flow states shock should result in a decrease, rather than an increase, of the PCO2 gap.
The major role of cardiac output in the value of ∆PCO2 was demonstrated in animal studies that compared ∆PCO2 changes between models of ischemic hypoxia and models of hypoxic hypoxia (28,29). Ischemic hypoxia was created by reducing blood flow using progressive bleeding in pigs (28) or in sheep (29). Hypoxic hypoxia was created either by a progressive reduction of inspired oxygen concentration (28) or by progressive intratracheal instillation of hydrochloric acid (29). In both studies, cardiac output remained unchanged in the hypoxic hypoxia group. Significantly, ∆PCO2 increased in the ischemic hypoxia group whereas it remained unchanged in the hypoxic hypoxia group (28,29). Similar results were reported by Vallet et al. in a model of vascular isolated dog hind limb (30). Indeed, ∆PCO2 significantly increased when limb hypoxia was induced by ischemia while it remained unchanged when hypoxia was induced by hypoxemia with maintained limb blood flow (30).
All these experimental (28-30) and clinical (25-27) studies have confirmed that during tissue hypoxia, ∆PCO2 can be either high or normal depending on cardiac output. Thus, a normal ∆PCO2 cannot exclude the absence of tissue hypoxia in high blood flow states. On the other hand, ∆PCO2 can be elevated in cases of low cardiac output, even in the absence of tissue hypoxia.
In summary, how to interpret the PCO2 gap in practice?
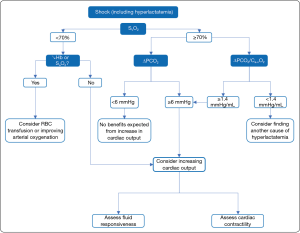
An increased PCO2 gap (>6 mmHg) suggests that cardiac output is not high enough with respect to the global metabolic conditions:
- In cases of shock (e.g., increased blood lactate), a high PCO2 gap could prompt clinicians to increase cardiac output with the aim of reducing tissue hypoxia (Figure 3);
- In the absence of shock, a high PCO2 gap can be associated with an increased oxygen demand.
In a patient with a high initial value of ∆PCO2, following the time-course of ∆PCO2 can also be helpful to assess the global metabolic effects of a therapy aiming at increasing cardiac output. Under conditions of oxygen supply-dependency, when cardiac output increases, the decrease in anaerobic metabolism tends to decrease ∆PCO2 but the increase in VO2 tends to increase ∆PCO2. As a result, ∆PCO2 is expected to decrease to a lesser extent than in the case of oxygen supply independence. Consequently, unchanged ∆PCO2 with therapy should not mean that the therapy has failed but rather that the treatment should be intensified until obtaining a frank decrease in ∆PCO2, indicating that the critical level of O2 delivery has been actually overcome.
On the other hand, a normal PCO2 gap (≤6 mmHg) suggests that cardiac output is high enough to wash out the amount of the CO2 produced from the peripheral tissues (Figure 2). Thus, increasing cardiac output has little chance to improve global oxygenation and such a strategy should not be a priority.
Combined analysis of ∆PCO2 and oxygen-derived variables
Even though ΔPCO2 cannot directly identify the presence of anaerobic metabolism, its combination with oxygen-derived variables has been suggested to overcome this issue (31). Indeed, as mentioned above, in case of anaerobic metabolism, VCO2 tends to increase because of the buffering of excessively produced protons, but also tends to decrease because of the decrease in VO2. Then, indexing VCO2 by VO2 should help detect the excess in CO2 produced due to the occurrence of anaerobic metabolism. In other words, dividing VCO2 by VO2 may help detect the production of CO2 which is not due to VO2.
The issue is then to estimate the ratio VCO2/VO2 at the bedside. As shown on Figure 4, using the Fick equation, and substituting CCO2 by PCO2, as suggested above, this ratio can be estimated by the ΔPCO2/Ca-vO2 ratio, where Ca-vO2 stands for the arteriovenous difference in O2 content.

In a series of 89 critically ill patients (148 measurements) where the mixed venous blood was sampled through a pulmonary catheter, a close correlation was found between blood lactate concentration and the ∆PCO2/Ca-vO2 ratio, while no correlation was found between blood lactate concentration and ∆PCO2 alone and between blood lactate concentration and Ca-vO2 alone (31). Similarly, in 51 septic shock patients, Monnet et al. showed a significant correlation between blood lactate and the ∆PCO2/Ca-vO2 ratio when the venous blood gas analysis was performed on the central, not the mixed venous blood (8). Similar results were found by Mesquida et al. who also demonstrated an increased mortality among patients with higher ∆PCO2/Ca-vO2 ratios, whereas no difference was observed for ∆PCO2 and ScvO2 (32).
In summary, an increase in the ∆PCO2/Ca-vO2 ratio above 1.4 mmHg/mL (31,32) should be considered as a marker of global anaerobic metabolism. Its normalization during resuscitation has been suggested as a therapeutic target (33). In the latter study, only lactate and ∆PCO2/Ca-vO2 resulted to be independently associated to mortality at multivariate analysis, among a series of haemodynamic variables in septic shock. Furthermore, mortality was significantly higher among patients with increase in both lactate and ∆PCO2/Ca-vO2, compared to the one of those with only elevated lactate levels and a normal ∆PCO2/Ca-vO2.
ScvO2vs. PCO2-derived indices
An advantage of the PCO2 gap over ScvO2 is that it remains a valid marker of the adequacy of cardiac output to the metabolic conditions even if the microcirculation is injured and the oxygen extraction is impaired. This could be due to the fact that CO2 is about 20 times more soluble than O2 (34). The microcirculatory impairment, with large venoarterial shunts, impedes the diffusion of O2 between cells and red blood cells, while the diffusion of CO2 remains unaltered (34). A confirmation comes from the study performed by Ospina-Tascón et al., where, in the early phases of septic shock, ∆PCO2 was actually able to detect the adequacy of microvascular blood flow (35).
Aiming at illustrating the superiority of the PCO2 gap over SvO2, Vallée et al. included 50 septic shock patients where a ScvO2 higher than 70% had been achieved (36). The central venous PCO2-arterial PCO2 difference (PCO2 gap) was abnormally high (>6 mmHg) in half of the patients (36). In that subgroup, blood lactate level tended to be higher and cardiac output to be lower compared to patients with a central PCO2 gap ≤6 mmHg. The authors concluded that ScvO2 may not be sufficient to guide therapy and that, when the 70% ScvO2 value is reached, the presence of a central PCO2 gap >6 mmHg might be useful to identify patients who still remain inadequately resuscitated (36). Another study showed that the combination of ScvO2 and central PCO2 gap predicted outcome in 172 critically ill patients resuscitated from septic shock better than ScvO2 alone (37). Patients who met both targets appeared to clear lactate more efficiently (37). Similar results were reported in a series of septic shock patients (38).
Regarding the comparison of ScvO2 with the central ∆PCO2/Ca-vO2 ratio, our team performed a study where 51 critically ill patients received fluid (8). In patients in whom volume expansion increased cardiac output, central PCO2 gap was able to follow the changes in cardiac output. Among patients in whom cardiac output increased, VO2 increased in around half of the cases (indicating dependency between VO2 and O2 delivery) while VO2 remained stable in the other ones (indicating independence between VO2 and O2 delivery). The increase of VO2 was detected by changes in the ∆PCO2/Ca-vO2 ratio but not by the changes in ∆PCO2 (8). Interestingly, in our cohort, ScvO2 could not detect changes in VO2, because it included a large proportion of septic shock patients in whom ScvO2 was in the normal range due to oxygen extraction impairment. This confirmed the superiority of the ∆PCO2/Ca-vO2 ratio over ScvO2 to detect tissue hypoxia in septic shock patients. Finally, the changes in lactate were also able to detect changes in VO2. However, lactate was measured three hours after fluid administration while the ∆PCO2/Ca-vO2 ratio was measured immediately after its end (8). This suggests that one advantage of the ∆PCO2/Ca-vO2 ratio over lactate is that it changes immediately after changes in VO2. However, Mallat et al. observed in septic shock patients that the increase in VO2 after volume expansion was detected much better by both the ∆PCO2/Ca-vO2 and the Cv-aCO2/Ca-vO2 ratio than by blood lactate (39).
In summary, all these arguments suggest that, in case of septic shock with O2 extraction impairment, in contrast with SvO2 or ScvO2, ∆PCO2remains a reliable marker of the adequacy of cardiac output with the metabolic condition and that the ∆PCO2/Ca-vO2 ratio remains a valid indicator of the adequacy between O2 delivery and VO2. Moreover, compared to lactate, the CO2-derived variables have the advantage to change without delay and to follow the metabolic condition in real time.
Errors and pitfalls of the PCO2 gap
Although many studies confirmed the association between an elevation in both ∆PCO2 and ∆PCO2/Ca-vO2 ratio and poor outcome in terms of lactate clearance, changes in VO2 and mortality (40-42), some other ones showed a limited or even a negative correlation between elevated ∆PCO2 and increase in blood lactate or mortality (43-45). Part of the discrepancy might be related to the fact that the latter studies were performed in post-cardiac surgery patients.
Haemodilution was recently investigated by Dubin et al. in an experimental model (46): the reliability of the ∆PCO2/Ca-vO2 ratio was compared between sheep with progressive haemorrhage and sheep with progressive haemodilution. Interestingly, the authors observed that in the haemodilution group, the ∆PCO2/Ca-vO2 ratio increased despite the absence of anaerobic metabolism. These findings, together with the high correlation with haemoglobin changes (R2=0.79; P<0.001), suggest that changes were explained by a rightward shift of the relationship between PCO2 and CCO2 (46).
In this regard, conflicting results have been reported also in terms of prognostic value of ∆PCO2/Ca-vO2 and ∆CCO2/Ca-vO2: while some authors observed that the ∆CCO2/Ca-vO2 ratio was an independent predictor of mortality, contrary to the ∆PCO2/Ca-vO2 ratio (33), others observed that the ∆PCO2/Ca-vO2 ratio but not the ∆CCO2/Ca-vO2 was associated with increased mortality (42).
Other authors investigated possible causes of misleading interpretation of both ∆PCO2 and the ∆PCO2/Ca-vO2 ratio. Mallat et al. showed that hyperventilation creates an increase in ∆PCO2 in healthy volunteers (47). Saludes et al. tested the effects of a hyperoxygenation trial on ∆PCO2 (48), and observed that, even though oxygen parameters increased both on the arterial and venous side, PCO2 augmented only in the venous blood, leading to an increase in both ∆PCO2 and ∆PCO2/Ca-vO2 ratio which was probably not related to changes in blood flow (48).
In addition, some technical aspects should be kept in mind when these indices are used in clinical practice. First, some errors in the PCO2 gap measurements may occur when sampling the venous blood: incorrect sample container, contaminated sample by air or venous blood or catheter fluid (49). Second, a too long delay of transport of blood sampling may significantly change the blood gas content at the venous and the arterial site (50).
Third, it is important to remind that variations in both ∆PCO2 and the ∆PCO2/Ca-vO2 ratio are submitted to a certain degree of variability. In this regard, in a series of 192 patients, Mallat et al. showed that the smallest detectable difference of ∆PCO2 was ±1.8 mmHg, corresponding to a least significant change of 32%. For the ∆PCO2/Ca-vO2 ratio, the smallest detectable difference was ±0.57 mmHg/mL, corresponding to a least significant change of 38% (51).
Conclusions
A proper analysis of the physiology of CO2 metabolism reveals that the PCO2 gap indicates the adequacy of cardiac output with the metabolic condition while the adequacy between O2 delivery and O2 consumption is better indicated by the ∆PCO2/Ca-vO2 ratio in critically ill patients. The CO2-derived indices seem to be quite reliable when measured in the central venous blood. In contrast to SvO2 or ScvO2, they remain useful in septic shock patients with an impaired O2 extraction.
Acknowledgments
None.
Footnote
Conflicts of Interest: JL Teboul and X Monnet are members of the Medical Advisory Board of Pulsion Medical Systems, Getinge. F Gavelli has no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Vincent JL, De Backer D. Oxygen transport-the oxygen delivery controversy. Intensive Care Med 2004;30:1990-6. [Crossref] [PubMed]
- Gattinoni L, Brazzi L, Pelosi P, et al. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med 1995;333:1025-32. [Crossref] [PubMed]
- Monnet X, Teboul JL. My patient has received fluid. How to assess its efficacy and side effects? Ann Intensive Care 2018;8:54. [Crossref] [PubMed]
- Londoño J, Niño C, Díaz J, et al. Association of Clinical Hypoperfusion Variables With Lactate Clearance and Hospital Mortality. Shock 2018;50:286-92. [Crossref] [PubMed]
- Legrand M, Payen D. Understanding urine output in critically ill patients. Ann Intensive Care 2011;1:13. [Crossref] [PubMed]
- Hernandez G, Bellomo R, Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med 2019;45:82-5. [Crossref] [PubMed]
- Vincent JL, Quintairos E, Silva A, et al. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care 2016;20:257. [Crossref] [PubMed]
- Monnet X, Julien F, Ait-Hamou N, et al. Lactate and venoarterial carbon dioxide difference/arterial-venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med 2013;41:1412-20. [Crossref] [PubMed]
- Zhang H, Vincent JL. Arteriovenous differences in PCO2 and pH are good indicators of critical hypoperfusion. Am Rev Respir Dis 1993;148:867-71. [Crossref] [PubMed]
- Randall HM, Cohen JJ. Anaerobic CO2 production by dog kidney in vitro. Am J Physiol 1966;211:493-505. [Crossref] [PubMed]
- Jensen FB. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol Scand 2004;182:215-27. [Crossref] [PubMed]
- Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev 2000;80:681-715. [Crossref] [PubMed]
- West J. Gas transport to the periphery: how gases are moved to the peripheral tissues. In: Respiratory physiology: the essentials. 4th edition. Baltimore: Williams and Wilkins, 1990:69-85.
- Teboul JL, Scheeren T. Understanding the Haldane effect. Intensive Care Med 2017;43:91-3. [Crossref] [PubMed]
- Douglas AR, Jones NL, Reed JW. Calculation of whole blood CO2 content. J Appl Physiol (1985) 1988;65:473-7. [Crossref] [PubMed]
- Cavaliere F, Giovannini I, Chiarla C, et al. Comparison of two methods to assess blood CO2 equilibration curve in mechanically ventilated patients. Respir Physiol Neurobiol 2005;146:77-83. [Crossref] [PubMed]
- Jensen FB. Comparative analysis of autoxidation of haemoglobin. J Exp Biol 2001;204:2029-33. [PubMed]
- McHardy GJ. The relationship between the differences in pressure and content of carbon dioxide in arterial and venous blood. Clin Sci 1967;32:299-309. [PubMed]
- Groeneveld AB, Vermeij CG, Thijs LG. Arterial and mixed venous blood acid-base balance during hypoperfusion with incremental positive end-expiratory pressure in the pig. Anesth Analg 1991;73:576-82. [PubMed]
- Teboul JL, Mercat A, Lenique F, et al. Value of the venous-arterial PCO2 gradient to reflect the oxygen supply to demand in humans: effects of dobutamine. Crit Care Med 1998;26:1007-10. [Crossref] [PubMed]
- Grundler W, Weil MH, Rackow EC. Arteriovenous carbon dioxide and pH gradients during cardiac arrest. Circulation 1986;74:1071-4. [Crossref] [PubMed]
- Weil MH, Rackow EC, Trevino R, et al. Difference in acid-base state between venous and arterial blood during cardiopulmonary resuscitation. N Engl J Med 1986;315:153-6. [Crossref] [PubMed]
- Dres M, Monnet X, Teboul JL. Hemodynamic management of cardiovascular failure by using PCO(2) venous-arterial difference. J Clin Monit Comput 2012;26:367-74. [Crossref] [PubMed]
- Van der Linden P, Rausin I, Deltell A, et al. Detection of tissue hypoxia by arteriovenous gradient for PCO2 and pH in anesthetized dogs during progressive hemorrhage. Anesth Analg 1995;80:269-75. [PubMed]
- Bakker J, Vincent JL, Gris P, et al. Veno-arterial carbon dioxide gradient in human septic shock. Chest 1992;101:509-15. [Crossref] [PubMed]
- Mecher CE, Rackow EC, Astiz ME, et al. Venous hypercarbia associated with severe sepsis and systemic hypoperfusion. Crit Care Med 1990;18:585-9. [Crossref] [PubMed]
- Wendon JA, Harrison PM, Keays R, et al. Arterial-venous pH differences and tissue hypoxia in patients with fulminant hepatic failure. Crit Care Med 1991;19:1362-4. [Crossref] [PubMed]
- Nevière R, Chagnon JL, Teboul JL, et al. Small intestine intramucosal PCO(2) and microvascular blood flow during hypoxic and ischemic hypoxia. Crit Care Med 2002;30:379-84. [Crossref] [PubMed]
- Dubin A, Murias G, Estenssoro E, et al. Intramucosal-arterial PCO2 gap fails to reflect intestinal dysoxia in hypoxic hypoxia. Crit Care 2002;6:514-20. [Crossref] [PubMed]
- Vallet B, Teboul JL, Cain S, et al. Venoarterial CO(2) difference during regional ischemic or hypoxic hypoxia. J Appl Physiol (1985) 2000;89:1317-21. [Crossref] [PubMed]
- Mekontso-Dessap A, Castelain V, Anguel N, et al. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med 2002;28:272-7. [Crossref] [PubMed]
- Mesquida J, Saludes P, Gruartmoner G, et al. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit Care 2015;19:126. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez W, et al. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O 2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med 2015;41:796-805. [Crossref] [PubMed]
- Vallet B, Pinsky MR, Cecconi M. Resuscitation of patients with septic shock: please “mind the gap”! Intensive Care Med 2013;39:1653-5. [Crossref] [PubMed]
- Ospina-Tascón GA, Umaña M, Bermúdez WF, et al. Can venous-to-arterial carbon dioxide differences reflect microcirculatory alterations in patients with septic shock? Intensive Care Med 2016;42:211-21. [Crossref] [PubMed]
- Vallée F, Vallet B, Mathe O, et al. Central venous-to-arterial carbon dioxide difference: an additional target for goal-directed therapy in septic shock? Intensive Care Med 2008;34:2218-25. [Crossref] [PubMed]
- Du W, Liu DW, Wang XT, et al. Combining central venous-to-arterial partial pressure of carbon dioxide difference and central venous oxygen saturation to guide resuscitation in septic shock. J Crit Care 2013;28:1110.e1-5. [Crossref] [PubMed]
- Mallat J, Pepy F, Lemyze M, et al. Central venous-to-arterial carbon dioxide partial pressure difference in early resuscitation from septic shock: a prospective observational study. Eur J Anaesthesiol 2014;31:371-80. [Crossref] [PubMed]
- Mallat J, Lemyze M, Meddour M, et al. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care 2016;6:10. [Crossref] [PubMed]
- Ospina-Tascón GA, Bautista-Rincón DF, Umaña M, et al. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care 2013;17:R294. [Crossref] [PubMed]
- He HW, Liu DW, Long Y, et al. High central venous-to-arterial CO2 difference/arterial-central venous O2 difference ratio is associated with poor lactate clearance in septic patients after resuscitation. J Crit Care 2016;31:76-81. [Crossref] [PubMed]
- Mesquida J, Saludes P, Pérez-Madrigal A, et al. Respiratory quotient estimations as additional prognostic tools in early septic shock. J Clin Monit Comput 2018;32:1065-72. [Crossref] [PubMed]
- Morel J, Grand N, Axiotis G, et al. High veno-arterial carbon dioxide gradient is not predictive of worst outcome after an elective cardiac surgery: a retrospective cohort study. J Clin Monit Comput 2016;30:783-9. [Crossref] [PubMed]
- Guinot PG, Badoux L, Bernard E, et al. Central Venous-to-Arterial Carbon Dioxide Partial Pressure Difference in Patients Undergoing Cardiac Surgery is Not Related to Postoperative Outcomes. J Cardiothorac Vasc Anesth 2017;31:1190-6. [Crossref] [PubMed]
- Abou-Arab O, Braik R, Huette P, et al. The ratios of central venous to arterial carbon dioxide content and tension to arteriovenous oxygen content are not associated with overall anaerobic metabolism in postoperative cardiac surgery patients. PloS One 2018;13:e0205950. [Crossref] [PubMed]
- Dubin A, Ferrara G, Kanoore Edul VS, et al. Venoarterial PCO2-to-arteriovenous oxygen content difference ratio is a poor surrogate for anaerobic metabolism in hemodilution: an experimental study. Ann Intensive Care 2017;7:65. [Crossref] [PubMed]
- Mallat J, Mohammad U, Lemyze M, et al. Acute hyperventilation increases the central venous-to-arterial PCO2 difference in stable septic shock patients. Ann Intensive Care 2017;7:31. [Crossref] [PubMed]
- Saludes P, Proença L, Gruartmoner G, et al. Central venous-to-arterial carbon dioxide difference and the effect of venous hyperoxia: A limiting factor, or an additional marker of severity in shock? J Clin Monit Comput 2017;31:1203-11. [Crossref] [PubMed]
- d’Ortho MP, Delclaux C, Zerah F, et al. Use of glass capillaries avoids the time changes in high blood PO(2) observed with plastic syringes. Chest 2001;120:1651-4. [Crossref] [PubMed]
- Wan XY, Wei LL, Jiang Y, et al. Effects of time delay and body temperature on measurements of central venous oxygen saturation, venous-arterial blood carbon dioxide partial pressures difference, venous-arterial blood carbon dioxide partial pressures difference/arterial-venous oxygen difference ratio and lactate. BMC Anesthesiol 2018;18:187. [Crossref] [PubMed]
- Mallat J, Lazkani A, Lemyze M, et al. Repeatability of blood gas parameters, PCO2 gap, and PCO2 gap to arterial-to-venous oxygen content difference in critically ill adult patients. Medicine (Baltimore) 2015;94:e415. [Crossref] [PubMed]