
Accuracy of endoscopic ultrasound in esophageal cancer staging
Introduction
In recent decades, endoscopic ultrasound (EUS) has emerged as an important tool in the evaluation and staging of esophageal cancer. It can provide detailed views of the esophageal wall layers and determine the depth of tumor invasion and surrounding lymph node metastases. The layers of the esophageal wall consist of the superficial mucosa, muscularis mucosa, submucosa, muscularis propria, and adventitia (Figure 1). When used in conjunction with cross sectional imaging, EUS can provide the necessary information to successfully stage esophageal malignancies. Proper staging provides information regarding prognosis and guides optimal treatment options.
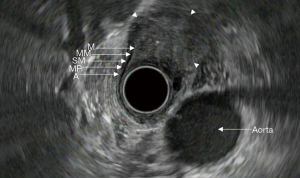
However, EUS is not without its limitations. EUS as a diagnostic tool is most reliable when performed by those with extensive training and practice; it is thus operator dependent. This leads to high inter-observer variability in those who do not regularly perform EUS. Furthermore, the echoendoscope is frequently unable to traverse stenotic lesions. This leads to inability to correctly assess the depth of tumor invasion. Finally, echoendoscopes are unable to reliably stage superficial malignancies confined to the mucosa or submucosa (1-4). Despite these shortcomings, EUS remains one of the most reliable methods available for locoregional staging of esophageal cancer.
The two predominant types of esophageal cancer are squamous cell carcinoma (SCC) and adenocarcinoma. Most cases in Western countries are due to adenocarcinoma arising from intestinal metaplasia of the distal esophagus. The incidence of adenocarcinoma has been increasing, which is thought to be related to rising rates of obesity (5). SCC, once the most common type of esophageal cancer in the United States in the 1960s, is now mainly seen in developing countries. Risk factors for SCC include smoking and alcohol use. SCC accounts for 87% of all esophageal cancers worldwide (5,6). Esophageal adenocarcinoma and SCC represent two different diseases with separate pathophysiology and prognoses. However, staging for both of these malignancies is determined similarly with a combination of endoscopic ultrasonography and cross-sectional imaging, as will be discussed.
Comparison of EUS to cross-sectional imaging
The initial step in cancer staging is identification of distant metastasis. Esophageal cancer predominantly metastasizes to the liver, lung, and bone (7). Available imaging modalities for detection of metastatic disease include computed tomography (CT) and positron emission tomography (PET), with integrated CT/PET being superior to PET alone (8).
Once distant metastases have been ruled out, establishing the extent of locoregional disease is the next step. This can be done either through EUS or cross-sectional imaging, such as CT or magnetic resonance imaging (MRI). Proper staging is critical as this will determine the ideal treatment approach. Treatment options include endoscopic resection, surgery, chemotherapy, and radiation.
Prior studies have shown EUS is superior for determining the depth of tumor invasion (T stage) and identification of regional lymph node spread (N stage) compared to CT (9-13). T stage accuracy for EUS ranges between 71–92% as compared to 42–60% for CT (11,14-17). Other studies have shown local N stage accuracy ranges between 64%-88% for EUS as compared to 51–82% for CT (9,10,12,14-20). For detection of distant metastases however, CT has an accuracy of 90% as compared to 70% for EUS (17). These studies have consistently shown that EUS is better for locoregional staging compared to CT alone. The combination of cross-sectional imaging and EUS has been shown to provide complimentary information for locoregional staging and detection of distant metastatic disease (9,12,13,17,21,22).
Utility of EUS for superficial cancer staging
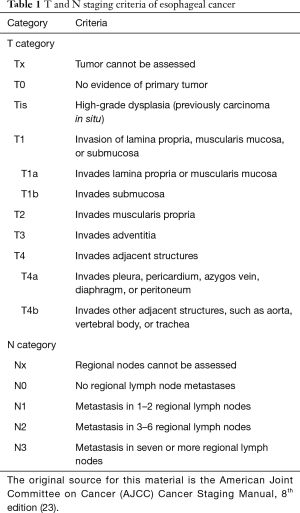
Superficial esophageal cancer is defined as malignancy limited to the mucosa or submucosa (Table 1). The prognosis and treatment options depend on tumor depth and regional lymph node involvement (24). The stage of esophageal cancer is the primary guide for treatment decisions.
Full table
Superficial esophageal cancer is defined as depth of tumor invasion between Tis (high-grade dysplasia) to T1b (submucosal invasion). Tis consists of pre-malignant cells confined to the epithelium that do not extend beyond the basement membrane. T1a tumors extend to the lamina propria, and T1b tumors invade into the submucosa. Esophageal cancer confined to the epithelium has a much more favorable prognosis than that extending into the submucosa. The submucosa contains lymphatic vessels, which promotes the spread of malignant cells to regional lymph nodes (25). In one case series involving 3,963 patients from the National Cancer Database (NCDB), the risk of lymph node metastasis was 5.0% for T1a and 16.6% for T1b lesions (26,27).
Given the difference in prognosis and treatment options, it is vital to make the distinction between mucosal (Tis-T1a) and submucosal cancer (T1b). Appropriate staging is essential because T1a esophageal cancer can be treated with endoscopic resection alone, however, T1b tumors usually require surgical resection such as esophagectomy (28-30). In superficial esophageal adenocarcinoma, endoscopic therapy can achieve complete remission in 94% of cases with a 5-year survival of 98% (31). Non-invasive imaging modalities such as CT and MRI are commonly used and can provide additional information about overall tumor size and regional lymph node involvement. However, both these imaging techniques are limited by the lack of ability to differentiate the layers of the esophageal wall (11,32). As previously mentioned, EUS can provide a detailed view of the layers of the esophageal wall. It has emerged as the imaging study of choice for locoregional staging of esophageal cancer due to its reliability and accessibility (33,34).
The overall accuracy of EUS for staging esophageal cancer ranges between 73% to 93%, depending on stage (35). The usefulness of EUS in staging superficial esophageal cancer has been a topic of debate, and its utility has been challenged by recent literature. A meta-analysis of 12 studies was conducted to evaluate whether EUS correctly predicts the T-stage of esophageal high-grade dysplasia or intramucosal adenocarcinoma compared to specimens obtained after endoscopic or surgical resection. EUS had a T-stage concordance of only 65%, which led to the conclusion it is not accurate for the staging of superficial esophageal cancer (1). Another meta-analysis of 19 studies was performed to determine the diagnostic accuracy of EUS in differentiating T1a from T1b esophageal cancer. It was found that EUS had a sensitivity and specificity of 85% and 87%, respectively, for T1a staging. The sensitivity and specificity were both 86% for T1b staging. The authors concluded that the accuracy of EUS for the staging of superficial esophageal cancer was adequate (2).
A retrospective cohort of 131 patients undergoing EUS for staging of early esophageal cancer was conducted to determine the usefulness of endosonography compared to pathologic staging of specimens removed with endoscopic mucosal resection. In 80% of cases, EUS did not demonstrate any submucosal involvement. However, evaluation of the endoscopically resected specimens revealed either submucosal invasion, positive resection margin for cancer, or lymphovascular invasion in 24% of these patients. In the remaining 20% of subjects, EUS findings were concerning for submucosal invasion or lymph node metastasis. However, in over half of these patients, there was no submucosal involvement on pathology. The authors concluded that EUS does not have any clinical impact on the work-up of superficial esophageal cancer and endoscopic resection plays the predominant role in diagnosis and staging (3).
A meta-analysis of 44 studies demonstrated that EUS had an overall T-stage accuracy of 79%. The pooled sensitivity and specificity for T1a tumors were 84% and 91%, respectively. While in T1b the pooled sensitivity and specificities were 83% and 89%, respectively. EUS was able to accurately differentiate between T1a and T1b tumors (4). While these studies demonstrate mixed findings and conclusions regarding the usefulness of EUS in the workup of superficial esophageal cancer, endoscopic resection undeniably gives more precise staging in addition to therapeutic benefit. Therefore, the value of EUS in these cases seems limited.
EUS and advanced cancer staging
Advanced malignancy is defined as any cancer that extends beyond the submucosa (T2 and greater). The ability to accurately evaluate the depth of tumor invasion can provide information regarding prognosis and guide treatment decisions. As previously discussed, EUS has questionable value in staging early esophageal cancer; however, accuracy increases with more advanced disease. EUS can accurately stage 67% of T1 tumors, 33% of T2 tumors, and 100% of T3 tumors. It is also significantly more sensitive for detecting T3 stage over early stage disease (36).
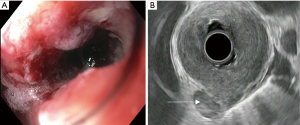
There are several challenges to using EUS for staging advanced cancers. EUS scopes are large caliber and measure over 12 mm in diameter. In obstructing tumors, the echoendoscope may not be able to traverse the malignant stricture and evaluate the depth of invasion. Between 20–36% of esophageal cancer patients present with a high-grade stricture that does not allow for complete passage of the echoendoscope (Figure 2A). This scenario precludes proper analysis of the cancer stage. The majority of obstructing tumors are seen in stage 3 and 4 cancers (37). For these lesions, there is significantly diminished accuracy in assessment of both T and N stage (28% and 72%, respectively) compared to fully traversable lesions (81% and 86%, respectively) (37). Options to improve diagnostic assessment include the use of dilators and through-the-scope ultrasound probes (miniprobe).
Among those with malignant esophageal strictures, dilation can be pursued if the lesion is partially obstructing. One study found that dilation to a range of 14 to 16 mm allowed for successful passage of the endoscope in 87% of cases. There were no reported complications (38). However, other studies with a more aggressive dilation regimen of up to 18 mm found high perforation rates of nearly 25% (37,39). Dilation can be accomplished by using a Savary-Gilliard® over-the-wire dilator or balloon. It is recommended to perform serial dilations over a period of several days to decrease the risk of perforation.
Another technique that can be considered in obstructing tumors is the use of miniprobe sonography. This is an ultrasound probe inserted through the biopsy channel of an endoscope. As the diameter of the miniprobe is under 3 mm, it can pass through most obstructing tumors. The higher frequencies of the miniprobe (up to 20 MHz) allow for a more detailed evaluation of the esophageal wall; however, the depth of penetration is less than the standard echoendoscope. Studies comparing miniprobe ultrasonography to standard endosonography have yielded mixed results. One study of 52 patients found that miniprobe sonography achieved higher accuracy rates for T-staging and similar rates for N-staging of stenosing esophageal cancers (40). Another article concluded that the miniprobe had similar accuracy compared to standard endosonography; however, the miniprobe was unable to reliably differentiate between T3 and T4 tumors. The accuracy for N-staging of traversable tumors was significantly better with the echoendoscope compared to the miniprobe (41).
In addition to difficulty with assessment of stenotic lesions, another limitation of EUS is staging after neoadjuvant chemotherapy. A recent meta-analysis revealed poor sensitivity and specificity for both T and N staging in patients undergoing EUS after having undergone chemotherapy. This is thought to be related to local inflammation and fibrosis caused by the chemotherapy or radiation. While chemoradiation treatment can decrease tumor size, it is not accompanied by restoration of the normal esophageal mucosa (42). This results in distortion of the layers of the esophageal wall with resultant erroneous staging.
EUS also plays a vital role in predicting tumor resectability. A previous study found that the accuracy of EUS to predict stages between T1–T3, which corresponded to R0 resectability, was 92% for adenocarcinoma of the distal esophagus. However, EUS predicted R0 resectability of SCC in only 66% of cases. The accuracy for T4 lesions was slightly lower at 80%, which was likely due to some of these lesions being non-traversable with the echoendoscope (18). An R0 resection margin is defined as the absence of tumor after surgical removal. Tumors that are classified as T4b are considered unresectable; however, T4a tumors can be considered for surgery providing there are no distant metastases (43). T4a tumors invade the pleura, pericardium, or diaphragm. T4b tumors are those invading any other adjacent structure, such as the mediastinum, trachea, or aorta. A safe resection margin is unable to be achieved with T4b tumors (44).
Lymph node staging
Lymph node evaluation is an essential component in the staging of esophageal carcinoma. This is particularly true in T2 disease, where the presence of lymph nodes may shift the treatment from surgical resection alone to include chemotherapy and radiation (45). EUS is the most accurate modality available for determination of locoregional lymphadenopathy. Endosonographic criteria suggestive of malignant lymph nodes include a hypoechoic sonographic pattern, sharply demarcated borders, round contours, and a width greater than 1 cm (Figure 2B). In contrast, benign lymph nodes tend to be smaller, elongated, hyperechoic, and have irregular borders. When these four endosonographic features are present, malignant lymph node involvement on histology can be predicted with up to 80–100% accuracy. Echogenicity is the most sensitive parameter for distinguishing benign from malignant nodes. However, only one-fourth of lymph nodes will have all four of these major features (46,47).
EUS can detect cervical, peri-esophageal and peri-gastric lymphadenopathy. The greater the number of malignant nodes, the worse the prognosis (10,48,49). In one study, 480 patients with esophageal carcinoma underwent esophagectomy, and there was a significant survival advantage in those with 2 or less malignant regional lymph nodes. The survival rate for 2 or less nodes was 20% at 5 years; however, those with greater than 2 nodes had 5% survival at 5 years (50). In a recent meta-analysis, the sensitivity and specificity for detecting celiac lymphadenopathy with EUS alone was 85% and 96%, respectively. The same study found that CT had a sensitivity of 42% and specificity of 93% for the detection of abdominal lymph node metastases.
The overall accuracy of EUS for nodal staging of esophageal cancer is 74% when used alone. This increases to nearly 90% when combined with fine-needle aspiration (FNA) (51). The specificity and accuracy of EUS-FNA is better than EUS alone; however, the sensitivities between the two tests are similar (52). When compared to CT, EUS-FNA has better sensitivity and accuracy (51). FNA improves accuracy as it allows for cytologic confirmation of metastatic disease. EUS-FNA is the least expensive strategy to obtain lymph node histology compared to CT guided FNA or surgery (53).
Inter-observer variability
EUS performance in esophageal cancer is operator-dependent and thus improves with increasing experience. A comparison of EUS performed at low-volume centers to those at high-volume centers demonstrated a lower sensitivity and specificity for T1 stage, T2 stage, and lymph node involvement in the low volume centers (54). An additional study over a 2-year period of 231 endosonographies performed for esophageal cancer staging by a single endoscopist determined that acceptable results could only be achieved after 100 examinations (55). The endosonographic T-stage was compared with the pathologic T-stage. For the first 100 endosonographies, the accuracy was 58%. However, the accuracy was significantly greater at 83% for the following 131 examinations.
Factors influencing variability among inexperienced operators include balloon over-inflation leading to difficulty in wall layer distinction, inflammatory extension of the tumor, and tangential imaging of stenotic tumors. Tumor stage also influences inter-observer variability. In one study, the agreement between inexperienced endosonographers was poor for all T stages; however, it was good for lymph node metastasis. Among experienced endosonographers, interobserver agreement was excellent for all T stages except T2 disease. Reproducibility of histologic confirmation of invasion depth for experienced operators was adequate for all stages except T1 lesions (56).
Conclusions
Correct locoregional staging by EUS in esophageal cancer is critical as this will determine appropriate treatment and disease prognosis. Cross-sectional imaging can be used for diagnosis of distant metastases. While EUS does have limitations, it has been shown to be the best diagnostic tool available to determine depth of invasion and local lymph node spread. Endoscopic resection can be both diagnostic and therapeutic for superficial cancers alone. In obstructing lesions that do not allow passage of the echoendoscope, dilation or use of a through-the-scope ultrasound catheter (miniprobe) can be utilized in order for staging to be completed. Inter-observer variability can be decreased by appropriate training and performance of EUS at high volume centers. EUS, when combined with FNA, has increased accuracy as compared to EUS or CT alone for regional lymph node staging. EUS, EUS-FNA, CT, and PET scan all work in conjunction with each other to identify the correct staging of esophageal cancer.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Young PE, Gentry AB, Acosta RD, et al. Endoscopic ultrasound does not accurately stage early adenocarcinoma or high-grade dysplasia of the esophagus. Clin Gastroenterol Hepatol 2010;8:1037-41. [Crossref] [PubMed]
- Thosani N, Singh H, Kapadia A, et al. Diagnostic accuracy of EUS in differentiating mucosal versus submucosal invasion of superficial esophageal cancers: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:242-53. [Crossref] [PubMed]
- Pouw RE, Heldoorn N, Alvarez Herrero L, et al. Do we still need EUS in the workup of patients with early esophageal neoplasia? A retrospective analysis of 131 cases. Gastrointest Endosc 2011;73:662-8. [Crossref] [PubMed]
- Luo LN, He LJ, Gao XY, et al. Endoscopic Ultrasound for Preoperative Esophageal Squamous Cell Carcinoma: a Meta-Analysis. PLoS One 2016;11:e0158373. [Crossref] [PubMed]
- Thrift AP. The epidemic of oesophageal carcinoma: Where are we now? Cancer Epidemiol 2016;41:88-95. [Crossref] [PubMed]
- Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev 2010;19:1468-70. [Crossref] [PubMed]
- Ai D, Zhu H, Ren W, et al. Patterns of distant organ metastases in esophageal cancer: a population-based study. J Thorac Dis 2017;9:3023-30. [Crossref] [PubMed]
- Bar-Shalom R, Guralnik L, Tsalic M, et al. The additional value of PET/CT over PET in FDG imaging of oesophageal cancer. Eur J Nucl Med Mol Imaging 2005;32:918-24. [Crossref] [PubMed]
- Takizawa K, Matsuda T, Kozu T, et al. Lymph node staging in esophageal squamous cell carcinoma: a comparative study of endoscopic ultrasonography versus computed tomography. J Gastroenterol Hepatol 2009;24:1687-91. [Crossref] [PubMed]
- van Vliet EP, Heijenbrok-Kal MH, Hunink MG, et al. Staging investigations for oesophageal cancer: a meta-analysis. Br J Cancer 2008;98:547-57. [Crossref] [PubMed]
- Lowe VJ, Booya F, Fletcher JG, et al. Comparison of positron emission tomography, computed tomography, and endoscopic ultrasound in the initial staging of patients with esophageal cancer. Mol Imaging Biol 2005;7:422-30. [Crossref] [PubMed]
- Vilgrain V, Mompoint D, Palazzo L, et al. Staging of esophageal carcinoma: comparison of results with endoscopic sonography and CT. AJR Am J Roentgenol 1990;155:277-81. [Crossref] [PubMed]
- Flamen P, Lerut A, Van Cutsem E, et al. Utility of positron emission tomography for the staging of patients with potentially operable esophageal carcinoma. J Clin Oncol 2000;18:3202-10. [Crossref] [PubMed]
- Tio TL, Cohen P, Coene PP, et al. Endosonography and computed tomography of esophageal carcinoma. Preoperative classification compared to the new (1987) TNM system. Gastroenterology 1989;96:1478-86. [Crossref] [PubMed]
- Grimm H, Binmoeller KF, Hamper K, et al. Endosonography for preoperative locoregional staging of esophageal and gastric cancer. Endoscopy 1993;25:224-30. [Crossref] [PubMed]
- Ziegler K, Sanft C, Zeitz M, et al. Evaluation of endosonography in TN staging of oesophageal cancer. Gut 1991;32:16-20. [Crossref] [PubMed]
- Botet JF, Lightdale CJ, Zauber AG, et al. Preoperative staging of esophageal cancer: comparison of endoscopic US and dynamic CT. Radiology 1991;181:419-25. [Crossref] [PubMed]
- Rösch T, Lorenz R, Zenker K, et al. Local staging and assessment of resectability in carcinoma of the esophagus, stomach, and duodenum by endoscopic ultrasonography. Gastrointest Endosc 1992;38:460-7. [Crossref] [PubMed]
- Grimm H, Soehendra N, Hamper K, et al. Endosonography versus computed tomography for determination of loco-regional spread in esophageal cancer: a prospective controlled study. In: Ferguson MK, Little AG, Skinner DB. editors. Diseases of the Esophagus, Vol 1: Malignant Diseases. Mount Kisco, NY: Futura, 1990:125-34.
- Dittler HJ, Bollschweiler E, Siewert JR. What is the value of endosonography in the preoperative staging of esophageal carcinoma? Dtsch Med Wochenschr 1991;116:561-6. [Crossref] [PubMed]
- Pfau PR, Perlman SB, Stanko P, et al. The role and clinical value of EUS in a multimodality esophageal carcinoma staging program with CT and positron emission tomography. Gastrointest Endosc 2007;65:377-84. [Crossref] [PubMed]
- Sandha GS, Severin D, Postema E, et al. Is positron emission tomography useful in locoregional staging of esophageal cancer? Results of a multidisciplinary initiative comparing CT, positron emission tomography, and EUS. Gastrointest Endosc 2008;67:402-9. [Crossref] [PubMed]
- Amin MB, American Joint Committee on Cancer, American Cancer Society. AJCC cancer staging manual. Eighth edition. Amin MB, Edge SB, Greene FL, et al. editors. Chicago IL: American Joint Committee on Cancer, Springer, 2017.
- Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer 1996;78:1820-8. [Crossref] [PubMed]
- Toh Y, Baba K, Ikebe M, et al. Endoscopic ultrasonography in the diagnosis of an early esophageal carcinoma. Hepatogastroenterology 1993;40:212-6. [PubMed]
- Merkow RP, Bilimoria KY, Keswani RN, et al. Treatment trends, risk of lymph node metastasis, and outcomes for localized esophageal cancer. J Natl Cancer Inst 2014.106. [PubMed]
- Endo M, Yoshino K, Kawano T, et al. Clinicopathologic analysis of lymph node metastasis in surgically resected superficial cancer of the thoracic esophagus. Dis Esophagus 2000;13:125-9. [Crossref] [PubMed]
- May A, Gossner L, Pech O, et al. Local endoscopic therapy for intraepithelial high-grade neoplasia and early adenocarcinoma in Barrett's oesophagus: acute-phase and intermediate results of a new treatment approach. Eur J Gastroenterol Hepatol 2002;14:1085-91. [Crossref] [PubMed]
- Crumley AB, Going JJ, McEwan K, et al. Endoscopic mucosal resection for gastroesophageal cancer in a U.K. population. Long-term follow-up of a consecutive series. Surg Endosc 2011;25:543-8. [Crossref] [PubMed]
- Pech O, Behrens A, May A, et al. Long-term results and risk factor analysis for recurrence after curative endoscopic therapy in 349 patients with high-grade intraepithelial neoplasia and mucosal adenocarcinoma in Barrett's oesophagus. Gut 2008;57:1200-6. [Crossref] [PubMed]
- Knabe M, May A, Ell C. Endoscopic therapy in early adenocarcinomas (Barrett's cancer) of the esophagus. J Dig Dis 2015;16:363-9. [Crossref] [PubMed]
- Aibe T, Fuji T, Okita K, et al. A fundamental study of normal layer structure of the gastrointestinal wall visualized by endoscopic ultrasonography. Scand J Gastroenterol Suppl 1986;123:6-15. [Crossref] [PubMed]
- Murata Y, Suzuki S, Ohta M, et al. Small ultrasonic probes for determination of the depth of superficial esophageal cancer. Gastrointest Endosc 1996;44:23-8. [Crossref] [PubMed]
- Fukuda M, Hirata K, Natori H. Endoscopic ultrasonography of the esophagus. World J Surg 2000;24:216-26. [Crossref] [PubMed]
- Rosch T. Endosonographic staging of esophageal cancer: a review of literature results. Gastrointest Endosc Clin N Am 1995;5:537-47. [Crossref] [PubMed]
- Shimpi RA, George J, Jowell P, et al. Staging of esophageal cancer by EUS: staging accuracy revisited. Gastrointest Endosc 2007;66:475-82. [Crossref] [PubMed]
- Catalano MF, Van Dam J, Sivak MV Jr. Malignant esophageal strictures: staging accuracy of endoscopic ultrasonography. Gastrointest Endosc 1995;41:535-9. [Crossref] [PubMed]
- Wallace MB, Hawes RH, Sahai AV, et al. Dilation of malignant esophageal stenosis to allow EUS guided fine-needle aspiration: safety and effect on patient management. Gastrointest Endosc 2000;51:309-13. [Crossref] [PubMed]
- Van Dam J, Rice TW, Catalano MF, et al. High-grade malignant stricture is predictive of esophageal tumor stage. Risks of endosonographic evaluation. Cancer 1993;71:2910-7. [Crossref] [PubMed]
- Menzel J, Hoepffner N, Nottberg H, et al. Preoperative staging of esophageal carcinoma: miniprobe sonography versus conventional endoscopic ultrasound in a prospective histopathologically verified study. Endoscopy 1999;31:291-7. [Crossref] [PubMed]
- Nesje LB, Svanes K, Viste A, et al. Comparison of a linear miniature ultrasound probe and a radial-scanning echoendoscope in TN staging of esophageal cancer. Scand J Gastroenterol 2000;35:997-1002. [Crossref] [PubMed]
- Sun F, Chen T, Han J, et al. Staging accuracy of endoscopic ultrasound for esophageal cancer after neoadjuvant chemotherapy: a meta-analysis and systematic review. Dis Esophagus 2015;28:757-71. [Crossref] [PubMed]
- Kosugi S, Ichikawa H, Kanda T, et al. Clinicopathological characteristics and prognosis of patients with esophageal carcinoma invading adjacent structures found during esophagectomy. J Surg Oncol 2012;105:767-72. [Crossref] [PubMed]
- Dittler HJ. Assessment of resectability of gastrointestinal cancers by endoscopic ultrasonography. Gastrointest Endosc Clin N Am 1995;5:569-75. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Adelstein DJ, et al. Role of clinically determined depth of tumor invasion in the treatment of esophageal carcinoma. J Thorac Cardiovasc Surg 2003;125:1091-102. [Crossref] [PubMed]
- Catalano MF, Sivak MV Jr, Rice T, et al. Endosonographic features predictive of lymph node metastasis. Gastrointest Endosc 1994;40:442-6. [Crossref] [PubMed]
- Bhutani MS, Hawes RH, Hoffman BJ. A comparison of the accuracy of echo features during endoscopic ultrasound (EUS) and EUS-guided fine-needle aspiration for diagnosis of malignant lymph node invasion. Gastrointest Endosc 1997;45:474-9. [Crossref] [PubMed]
- Twine CP, Roberts SA, Rawlinson CE, et al. Prognostic significance of the endoscopic ultrasound defined lymph node metastasis count in esophageal cancer. Dis Esophagus 2010;23:652-9. [Crossref] [PubMed]
- Chen J, Xu R, Hunt GC, et al. Influence of the number of malignant regional lymph nodes detected by endoscopic ultrasonography on survival stratification in esophageal adenocarcinoma. Clin Gastroenterol Hepatol 2006;4:573-9. [Crossref] [PubMed]
- Rice TW, Blackstone EH, Rybicki LA, et al. Refining esophageal cancer staging. J Thorac Cardiovasc Surg 2003;125:1103-13. [Crossref] [PubMed]
- Vazquez-Sequeiros E, Wiersema MJ, Clain JE, et al. Impact of lymph node staging on therapy of esophageal carcinoma. Gastroenterology 2003;125:1626-35. [Crossref] [PubMed]
- Wiersema MJ, Vilmann P, Giovannini M, et al. Endosonography-guided fine-needle aspiration biopsy: diagnostic accuracy and complication assessment. Gastroenterology 1997;112:1087-95. [Crossref] [PubMed]
- Harewood GC, Wiersema MJ. A cost analysis of endoscopic ultrasound in the evaluation of esophageal cancer. Am J Gastroenterol 2002;97:452-8. [Crossref] [PubMed]
- van Vliet EP, Eijkemans MJ, Poley JW, et al. Staging of esophageal carcinoma in a low-volume EUS center compared with reported results from high-volume centers. Gastrointest Endosc 2006;63:938-47. [Crossref] [PubMed]
- Fockens P, Van den Brande JH, van Dullemen HM, et al. Endosonographic T-staging of esophageal carcinoma: a learning curve. Gastrointest Endosc 1996;44:58-62. [Crossref] [PubMed]
- Catalano MF, Sivak MV Jr, Bedford RA, et al. Observer variation and reproducibility of endoscopic ultrasonography. Gastrointest Endosc 1995;41:115-20. [Crossref] [PubMed]


