
Video-assisted thoracoscopic surgery for invasive pulmonary fungal infection in haematology patients
Introduction
Fungal infection is a common complication in the treatments of haematological patients. It may be caused by the immunosuppression which was arose from the underlying disease itself, chemotherapy treatments or hematopoietic stem cell transplantation (HSCT). Especially in patients with persistent neutropenia after chemotherapy or HSCT, fungal infection is more likely to occur.
The most common form of fungal infection is invasive pulmonary aspergillosis (1). Although great progress has been made in diagnosis and treatments of invasive pulmonary aspergillosis in recent years, invasive pulmonary aspergillosis is still the leading cause of infectious pneumonia-related mortality in immunocompromised hosts (2,3). Allogeneic HSCT, neutropenia and graft versus host disease (GVHD) are the greatest risk factors (4).
One study demonstrated that the mortality rate associated with invasive fungal infection in HSCT recipients with active fungal infection was 40%, compared with 2.54% in HSCT recipients without history of invasive fungal infection (5). Therefore, the successful treatments of pulmonary fungal infection have great impacts on prognosis of haematological patients. However, it is difficult to eradicate the fungal infection with antifungal agent therapy alone in practice (6). Many haematological patients were delayed to receive HSCT to treat their underlying haematological diseases due to the insufficient antifungal treatments.
For those haematological patients with pulmonary fungal infections who failed with antifungal therapies or urged for HSCT, surgical treatments may serve as another treatment. In addition, surgical treatments may also be an appropriate solution for hemoptysis among haematological patients with invasive pulmonary fungal infections (7). Although surgical treatments may have to face many technical difficulties and high risks of postoperative complications, the outcomes of surgical treatments can be acceptable if the patients were carefully selected (8,9). Compared with traditional thoracic surgery, video-assisted thoracoscopic surgery (VATS) has the advantages of less surgical trauma and more flexible operative procedures. However, only a few series of studies reported the outcomes of VATS in the treatments of haematological patients with invasive pulmonary fungal infections. More studies are needed to clarify the safety and effectiveness of VATS in the treatments of haematological patients with invasive pulmonary fungal infections.
This study focused on the clinical outcomes and surgical procedures of invasive pulmonary fungal infections in haematological patients. Related potential influencing factors were analyzed to evaluate the safety and effectiveness of VATS in the treatments of invasive pulmonary fungal infection in haematological patients.
Methods
This retrospective study was conducted at The First Affiliated Hospital of Soochow University from January 2011 to December 2017. The purpose of this study was to evaluate the safety and effectiveness of VATS in the treatments of invasive pulmonary fungal infection in haematological patients by analyzing related factors. Factors we considered included general condition, type of haematological diseases, preoperative clinical symptoms, surgical procedures, length of postoperative hospital stay, incidence of postoperative complications and postoperative follow-up. Approval for the study was obtained from the Institutional Review Board of the hospital.
The preliminary diagnosis of invasive pulmonary fungal infection was made on according to the clinical, imaging and mycological manifestations. The clinical manifestations include fever, chest pain, cough or hemoptysis. The imaging findings include computed tomography (CT) images of pathognomonic crescent or halo signs. The mycological diagnosis includes serum galactomannan (GM) tests and sputum culture. The final diagnosis of pulmonary invasive fungal infection was confirmed by histopathological examination of resected specimens.
All patients were treated with antifungal drugs for an average of 8 weeks after making preliminary diagnosis. Those patients with failed antifungal agents therapies, recurrent hemoptysis, localized lesion and needs for definitive diagnosis and treatments before receiving chemotherapy or HSCT were selected as the appropriate targets for surgical treatments.
The timing of surgical treatments needs to be chosen carefully. In principle, the timing of surgical treatments should be chosen in the interval of chemotherapy or the recovery stage of bone marrow. Biochemical and blood routine examination were performed before operation. The preoperative indexes were adjusted by intermittent blood transfusion or bone marrow stimulation to meet the following criteria: (I) white blood cell counts >3×109/L; (II) neutrophil counts >0.5×109/L; (III) blood platelet counts >50×109/L and (IV) hemoglobin counts >60 g/L. If patients don’t meet these criteria, surgical treatments would be a high-risk choice and they would be excluded. In addition, patients with fever, pneumonia or rejection to surgical treatments were also excluded.
The choice of surgical procedure was based on the location and range of lesion. Video-assisted thoracoscopic wedge resection was chosen when the lesion met the following criteria: (I) localized unilateral lesion in outer one-third of the peripheral lung; (II) less than 3 cm in size; and (III) a safe resection margin greater than 1 cm can be achieved. Video-assisted thoracoscopic segmentectomy was chosen when the lesion located within a single segment which was hard to remove completely by wedge resection. Video-assisted thoracoscopic lobectomy was suitable for patients with overlapped or extensive lesion which was hard to remove completely by wedge resection or segmentectomy.
All patients were treated with antifungal drugs after surgery. Patients would be discharged from hospital or transferred to haematology department to receive further treatments when they recovered well after operation.
All patients were followed up for 6 to 24 months after surgery. The follow-up data were obtained from outpatient clinic chart reviews or by telephone calls to patients or family. The incidence of disease recurrence and follow-up treatments were collected and analyzed.
Results
As showed in Table 1, a total of 51 haematological patients with invasive pulmonary fungal infection were involved in this study. There were 27 males and 24 females with an average age of 35 years. The most common haematological disease was acute lymphoblastic leukemia in 30 patients, followed by acute myeloblastic leukemia in 9 patients.

Full table
The most common preoperative symptom before operation was fever, which occurred in 43 patients. Cough occurred in 22 patients, followed by chest pain which occurred in 17 patients and hemoptysis occurred in 7 patients. CT chest scan showed halo sign in 27 patients and crescent sign in 20 patients. However, there were 4 patients only showed diffuse infiltration on CT image. There were 5 patients showed positive sputum culture results and 10 patients showed positive results of serum GM tests.
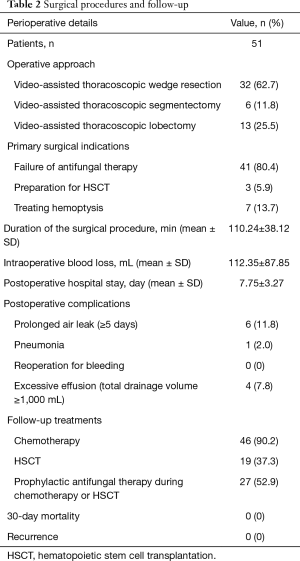
Surgery details are summarized in Table 2. Of these 51 patients, 32 patients underwent video-assisted thoracoscopic wedge resection, 6 patients underwent video-assisted thoracoscopic segmentectomy and 13 patients underwent video-assisted thoracoscopic lobectomy, none of them was converted to open surgery. The failure of antifungal therapy was the main reason for surgical treatments in 41 patients. Three patients underwent surgical treatments for the purpose of HSCT. Seven patients received surgical treatments to treat hemoptysis. The mean duration of the surgical procedure was 110.24±38.12 min. The mean intraoperative blood loss was 112.35±87.85 mL. The mean postoperative hospital stay was 7.75±3.27 days. The most common postoperative complication was prolonged air leak which was found in 6 patients.
Full table
The follow-up data showed that all patients underwent further treatments after discharged from hospital or transferred to haematology department. Forty-six patients received chemotherapy and 19 patients received HSCT after surgical treatments. Of these patients, 27 patients underwent prophylactic antifungal treatments during chemotherapy or HSCT. No recurrence was observed during follow-up.
Discussion
Pulmonary invasive fungal infections are associated with high mortality rates in the immunocompromised host, especially in patients with acute leukaemia received induction chemotherapy as well as in HSCT patients (10). In these patients, aspergillus was the main cause of fungal infection (11). The global burden of invasive aspergillosis has recently estimated to be between 20,000 and 400,000 cases per year, with a mortality rate of 30–85% (12). Therefore, early diagnosis, active anti-fungal therapies and elective surgical treatments were important and may improve the prognosis and survival rate.
Diagnosis of pulmonary fungal infection in haematological patients is not straightforward. Chest CT scan played an important role in the diagnosis of invasive pulmonary fungal infection. The halo, a nodule with surrounding ground-glass opacity, is the most specific sign. However, well-shaped nodules without halo, air-crescent signs, and new cavities are also highly suggestive of invasive fungal infection (13,14). However, the signs are non-specific and some non-infectious conditions such as haemorrhage, congestive heart failure or haematological lesions have similar appearances. Therefore, only 85 per cent of patients could acquire early diagnosis by CT scan. The application of bronchoscopy (30–50% sensitivity) and percutaneous or endoscopic biopsy (35–80% sensitivity) was also useful, although the scope of application was limited (15,16).
Empirical antifungal treatments were commonly used in haematological patients with invasive pulmonary fungal infection. Even so, some patients still have a poor prognosis due to the unsuccessful antifungal treatments. When antifungal treatments failed, a medical-surgical approach may be beneficial for these haematological patients. As reported in literature, if patients with fairly localized lesions showed resistance to antifungal treatments, but urged for chemotherapy or HSCT, active surgical treatments may be beneficial (17).
When to take surgical treatments is still a controversial issue in current literatures (18). In patients with hemoptysis, the morbidity and mortality rates of emergency pulmonary resection are high. As Andréjak et al. reported, the mortality rate of emergency pulmonary resection was high up to 35% (19). By contrast, the mortality rates of pulmonary resection after bleeding controlled and planned pulmonary resection were 4% and 0%, separately (20). This study indicated the significance of avoiding emergent lung resection as much as possible to minimize the morbidity and mortality. In asymptomatic patients, whether surgical treatments were necessary still remained an open question. Prophylactic lung resection to prevent massive hemoptysis was mentioned in recent years literatures (21). However, preoperative antifungal treatments were necessary. Pidhorecky et al. reported that surgical treatments could be considered when CT image showed the lesion was limited and unchanged after 2 weeks of antifungal treatments (7). We thought surgical treatments should be considered if the lesion was limited and showed resistance to antifungal treatments. Surgical treatments in remission stage of chemotherapy were relatively safe and suitable for haematological patients. However, surgical treatments in the periods of granulocytopenia should considered seriously, due to the high risk of surgical procedure, high incidence of postoperative complications and high mortality (22). Emergency surgical treatments should be avoid as much as possible because of the high risk of bleeding. Only in the case of severe hemoptysis, emergency surgical treatments need to be considered. Because of the haematological diseases, the surgical methods of these haematological patients was different from other patients. These haematological patients were characterized by high-risk of blooding and low immunity. Therefore, the surgical procedure should be considered seriously for the purposes of removing lesion completely while reducing the surgical trauma and shortening the operative time.
The ideal surgical treatments were anatomical resections to remove the lesion and potential cavity completely (18). Because the residual lesion was the potential factor of recurrence. Lobectomy was considered as the first choice and it was proved feasible anatomically and functionally (23). In some cases, segmentectomy and wedge resection was also proved feasible in haematological patients with invasive pulmonary fungal infection (20,24). Pneumonectomy should be considered seriously due to the high risk of serious postoperative complications, especially in emaciated patients with marginal spirometric values (25,26). In this study, the choices of surgical methods were based on the location and scope of lesions for the aim of controlling the clinical symptoms, guiding medical therapies and improving the qualities of life on the basis of removing the lesion completely and maximally reserving the lung function.
Thoracoscopic treatment of pleural adhesion and fixed lymph nodes was always a difficult problem in patients with inflammatory diseases. Most haematological patients are young and take corticosteroids to treat their haematological diseases, therefore the pleural adhesion in haematological patients was much less than other patients and fixed lymph nodes were not seen commonly. Corticosteroids have the function of controlling inflammation and reducing the formation of pleural adhesion (27). Therefore, the management of pleural adhesion and fixed lymph nodes in haematological patients were not difficult. Thoracoscopy can magnify images and explore thorax completely. If pleural adhesions were found during operation, thoracoscopy can deal with these pleural adhesions easily and safely.
VATS has developed rapidly in recent years and brought great changes to thoracic surgery. It can achieve same goals as traditional operation with less operative trauma and more elaborate operative procedures. Haematological patients have the characteristics of high-risk blooding and low immunity. Compared with traditional open chest surgery, VATS showed great advantages of less blood loss, less operative trauma and quick postoperative recovery. Most haematological patients with invasive pulmonary fungal infection were in young-middle age and have well cardiopulmonary function. Additionally, most patients have no chronic respiratory diseases such as emphysema and tuberculosis. Therefore, most patients can tolerate the surgical procedure well. Furthermore, the difficulties of surgical procedure in haematological patients are relatively easier because of the low proportion of pleural adhesion. For these reasons, the proportions of intraoperative and postoperative complications are much lower than traditional operations. Therefore, VATS maybe more beneficial for thrombocytopenic and neutropenic patients.
The management of potential complications is also an important aspect. Due to the low immunity of haematological patients, once serious complications occurred, it may bring serious consequences to haematological patients. A propriate surgical procedure could remove lesions while protect normal pulmonary tissue. Reducing intraoperative blood loss and shortening operative time are all important measures to reduce the incidence of complications. It is also very important to observe closely after operation, to detect and deal with problems as early as possible. In our study, the overall proportion of postoperative complications was 21.6%. Of all these postoperative complications, excessive effusion and prolonged air leak were most common seen. Prolonged air leak all occurred in patients with video-assisted thoracoscopic wedge resection. This suggested that the lung tissue should be sutured to reduce the proportions of postoperative complications in patients with video-assisted thoracoscopic wedge resection.
Whether postoperative antifungal therapies are necessary or not is another question needs further research. Ho and Calandra reported that the use of prophylactic antifungal agents in high-risk surgical patients was associated with a reduction in the proportion of patients with candida and fungal infections (28,29). Yuan et al. reported that the intraoperative povidone-iodine lavage combined with postoperative antifungal pharmacotherapy likely contributes to the favorable long-term outcomes (30). However, Sagan and Pakyz have reported that perioperative antifungal pharmacotherapy did not appear to improve the outcomes of surgical treatment for pulmonary aspergilloma, but may cause renal dysfunction (31,32). We thought haematological patients with neutropenia or in the preparation of HSCT should receive postoperative antifungal pharmacotherapy until the granulocyte level return to normal. For patients with normal granulocyte level, the doses of antifungal drugs should be individual.
Limitation
Several limitations of this study should be acknowledged. First of all, the retrospective design of this study may cause the possibility of some inevitable selection bias. Secondly, these haematological patients who underwent surgery were selected seriously. Thirdly, this report is from a single institution. Further research is needed to validate the safety and effectiveness of VATS for invasive pulmonary fungal infection.
Conclusions
VATS is effective and feasible in the treatment of invasive fungal infection among patients with haematological disease. The follow-up of surgical treatments showed good results, preoperative symptoms were relieved in most patients, and no recurrence occurred.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Approval for the study was obtained from the Institutional Review Board of the hospital.
References
- Segal BH. Aspergillosis. N Engl J Med 2009;360:1870-84. [Crossref] [PubMed]
- Heldt S, Eigl S, Prattes J, et al. Levels of interleukin (IL)-6 and IL-8 are elevated in serum and bronchoalveolar lavage fluid of haematological patients with invasive pulmonary aspergillosis. Mycoses 2017;60:818-25. [Crossref] [PubMed]
- Cornely OA, Lass-Flörl C, Lagrou K, et al. Improving outcome of fungal diseases - Guiding experts and patients towards excellence. Mycoses 2017;60:420-25. [Crossref] [PubMed]
- Kriengkauykiat J, Ito JI, Dadwal SS. Epidemiology and treatment approaches in management of invasive fungal infections. Clinical Epidemiology 2011;3:175-91. [PubMed]
- Aki ZS, Sucak GT, Yeğin ZA, et al. Hematopoietic Stem Cell Transplantation in Patients With Active Fungal Infection: Not a Contraindication for Transplantation. Transplant Proc 2008;40:1579-85. [Crossref] [PubMed]
- Denning DW, Ribaud P, Milpied N, et al. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis 2002;34:563-71. [Crossref] [PubMed]
- Pidhorecky I, Urschel J, Anderson T. Resection of invasive pulmonary aspergillosis in immunocompromised patients. Ann Surg Oncol 2000;7:312-7. [Crossref] [PubMed]
- Moon Y, Park JK, Sung SW. Surgery for localized pulmonary mycotic infections in patients with hematopoietic disorder. J Cardiothorac Surg 2015;10:91. [Crossref] [PubMed]
- Chretien ML, Legouge C, Pagès PB, et al. Emergency and elective pulmonary surgical resection in haematological patients with invasive fungal infections: a report of 50 cases in a single centre. Clin Microbiol Infect 2016;22:782-7. [Crossref] [PubMed]
- Atalla A, Garnica M, Maiolino A, et al. Risk factors for invasive mold diseases in allogeneic hematopoietic cell transplant recipients. Transpl Infect Dis 2015;17:7-13. [Crossref] [PubMed]
- Pergam SA. Fungal Pneumonia in Patients with Hematologic Malignancies and Hematopoietic Cell Transplantation. Clin Chest Med 2017;38:279-94. [Crossref] [PubMed]
- Heinz WJ, Vehreschild JJ, Buchheidt D. Diagnostic work up to assess early response indicators in invasive pulmonary aspergillosis in adult patients with haematologic malignancies. Mycoses 2019;62:486-93. [Crossref] [PubMed]
- Kubo T, Ohno Y, Takenaka D, et al. Standard-dose vs. low-dose CT protocols in the evaluation of localized lung lesions: Capability for lesion characterization—iLEAD study. Eur J Radiol Open 2016;3:67-73. [Crossref] [PubMed]
- Prasad A, Agarwal K, Deepak D, et al. Pulmonary Aspergillosis: What CT can Offer Before it is too Late! J Clin Diagn Res 2016;10:TE01-05. [PubMed]
- Shi JM, Cai Z, Huang H, et al. Role of CT-guided percutaneous lung biopsy in diagnosis of pulmonary fungal infection in patients with hematologic diseases. Int J Hematol 2009;89:624-7. [Crossref] [PubMed]
- Lass-Flörl C, Resch G, Nachbaur D, et al. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin Infect Dis 2007;45:e101-4. [Crossref] [PubMed]
- Komori K, Hattori A, Matsunaga T, et al. Feasibility of surgery for pulmonary aspergilloma: analysis of the operative modes. Gen Thorac Cardiovasc Surg 2018;66:276-83. [Crossref] [PubMed]
- El Hammoumi MM, Slaoui O, El OF, et al. Lung resection in pulmonary aspergilloma: experience of a Moroccan center. BMC Surgery 2015;15:114. [Crossref] [PubMed]
- Andréjak C, Parrot A, Bazelly B, et al. Surgical lung resection for severe hemoptysis. Ann Thorac Surg 2009;88:1556-65. [Crossref] [PubMed]
- Ichinose J, Kohno T, Fujimori S. Video-assisted thoracic surgery for pulmonary aspergilloma. Interact Cardiovasc Thorac Surg 2010;10:927-30. [Crossref] [PubMed]
- Cesar JM, Resende JS, Amaral NF, et al. Cavernostomy x Resection for Pulmonary Aspergilloma: A 32-Year History. J Cardiothorac Surg 2011;6:129. [Crossref] [PubMed]
- Matt P, Bernet F, Habicht J, et al. Predicting outcome after lung resection for invasive pulmonary aspergillosis in patients with neutropenia. Chest 2004;126:1783-8. [Crossref] [PubMed]
- Lejay A, Falcoz PE, Santelmo N, et al. Surgery for aspergilloma: time trend towards improved results? Interact Cardiovasc Thorac Surg 2011;13:392-5. [Crossref] [PubMed]
- Aydoğdu K, İncekara F, Şahin MF, et al. Surgical management of pulmonary aspergilloma: clinical experience with 77 cases. Turk J Med Sci 2015;45:431-7. [Crossref] [PubMed]
- Shiraishi Y, Katsuragi N, Nakajima Y, et al. Pneumonectomy for complex aspergilloma: is it still dangerous? Eur J Cardiothorac Surg 2006;29:9-13. [Crossref] [PubMed]
- Kasprzyk M, Pieczyński K, Mania K, et al. Surgical treatment for pulmonary aspergilloma - early and long-term results. Kardiochir Torakochirurgia Pol 2017;14:99-103. [Crossref] [PubMed]
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. Br J Pharmacol 2006;148:245-54. [Crossref] [PubMed]
- Ho KM, Lipman J, Dobb GJ, et al. The use of prophylactic fluconazole in immunocompetent high-risk surgical patients: a meta-analysis. Crit Care 2005;9:R710-7. [Crossref] [PubMed]
- Calandra T, Marchetti O. Clinical Trials of Antifungal Prophylaxis among Patients Undergoing Surgery. Clin Infect Dis 2004;39 Suppl 4:S185-92. [Crossref] [PubMed]
- Yuan P, Cao JL, Huang S, et al. Sublobar Resection for Pulmonary Aspergilloma: A Safe Alternative to Lobectomy. Ann Thorac Surg 2017;103:1788-94. [Crossref] [PubMed]
- Sagan D, Goździuk K. Surgery for pulmonary aspergilloma in immunocompetent patients: no benefit from adjuvant antifungal pharmacotherapy. Ann Thorac Surg 2010;89:1603-10. [Crossref] [PubMed]
- Pakyz A, Bearman G. Adverse drug events complicate antifungal therapy for pulmonary aspergilloma. Consult Pharm 2008;23:804-8. [Crossref] [PubMed]


