
Clinical importance of Bcl-2-like 11 deletion polymorphism in idiopathic pulmonary fibrosis
Introduction
Idiopathic pulmonary fibrosis (IPF) is the most common form of idiopathic interstitial pneumonia (IP) associated with the histological pattern of usual interstitial pneumonia (UIP). IPF is characterized by a steady decline in pulmonary function, and median survival is approximately 3 years from diagnosis (1). Some IPF patients develop acute respiratory disease, called acute exacerbation (AE)-IPF (1-3), which can result in death within a few weeks to several months (1-3). In a sustained state of chronic inflammation, like IPF, glutathione drastically decreases and results in redox imbalance. However, reactive oxygen species (ROS) and peroxidic substances generated by cytotoxic mechanisms cannot be satisfactorily treated (4-8). ROS can damage alveolar epithelial cells and have an important role in inducing TGF-1 signaling that results in collagen-1 synthesis and fibrogenesis in pulmonary fibrosis (9-11). ROS are important in the fibrotic process in bleomycin-induced pulmonary fibrosis in humans and animals with IPF. Absence of ROS in mice lacking the p47phox subunit (Nox) 12 of NADPH oxidase protected the mice from bleomycin-induced pulmonary fibrosis (10,12). IPF is thought to cause fibrosis through alveolar epithelial injury and repetition of inappropriate repair, and apoptosis is reported to be involved in this condition (8,13).
Bcl-2-like 11 (BIM) is a pro-apoptotic member of the B cell CLL/lymphoma 2 protein family and is an important modulator of apoptosis (14,15). BIM expression is important in the activation of ROS overproduction via forkhead box O3 (FOXO3) (16). Recently, Ng et al. (17) reported a common intron deletion polymorphism in the gene encoding BIM. This polymorphism switched BIM splicing from exon 4 to exon 3, which resulted in expression of a BIM isoform lacking the pro-apoptotic B cell CLL/lymphoma 2 homology domain 3 (BH3). BIM deletion polymorphism has a silent single-nucleotide polymorphism (SNP) in exon 5 (c465C>T) and a deletion site (2,903 bp). Because IPF patients with this BIM deletion polymorphism do not have the BH3 domain that is critical in apoptosis, alveolar epithelial apoptosis and ROS production might be less robust in these patients than in IPF patients without this polymorphism.
We investigated the clinical features of IPF patients with and without the BIM deletion polymorphism and examined the importance of redox status.
Methods
Study population
We reviewed the medical records of 63 patients with IPF to determine their BIM deletion polymorphism status, which was determined by polymerase chain reaction (PCR) analysis of a peripheral blood specimen (serum 5 mL). The patients were treated in our department during the period from January 2006 through April 2018. Patient background characteristics, IP markers, respiratory function test results, and frequency of AE-IPF were compared in patients with and without BIM deletion polymorphism, as were total blood glutathione (tGSH), oxidized glutathione (GSSG), reduced glutathione (GSH), 8-hydroxy-2'-deoxyguanosine (8-OHdG), and 8-isoprostane (8-iso). Patient samples were stored at −80 °C until laboratory analysis.
This retrospective study was approved by the Institutional Review Board of Toho University Medical Center Omori Hospital (25-5).
Diagnosis of IPF
These patients were defined as having UIP patterns or non-UIP patterns on computed tomography (CT), and the diagnosis was confirmed by histological examination of lung biopsy specimens or the presence of findings consistent with the clinical diagnostic criteria for IPF (18). According to the official ATS/ERS/JRS/ALAT Clinical Practice Guideline, IPF is diagnosed when known causes of fibrosis (e.g., connective tissue disease or asbestos) are excluded and high-resolution CT shows a probable or definite UIP pattern. We re-evaluated IPF diagnoses in all patients at the time this study was performed, to ensure that initial diagnoses were correct.
Definition of AE-IPF
In accordance with the guidelines of the International Working Group Report (1), AE-IPF was diagnosed by using the following criteria: (I) worsening breathlessness within 30 days; (II) a diagnosis of IPF; (III) presence of new ground-glass opacities or consolidation on imaging; (IV) absence of hyperpigmentation shadows on CT. Patients with obvious pneumothorax, pulmonary embolism, or heart failure were excluded. Fatal AE-IPF was defined as AE-IPF resulting in death within 1 month after AE-IPF (19).
PCR analysis of BIM gene deletion polymorphism
To detect BIM deletion polymorphism, we performed PCR analysis, using the method of Ng et al. (17). A single primer set containing the deletion region of intron 2 was used, and two separate primer sets were designed for wild-type and deletion alleles. DNA was subjected to PCR amplification by using a primer designed to detect the deletion site (2,903 bp) of intron 2 of the BCL2L11 gene. PCR products obtained from the deletion (1,285 bp) and wild-type (4,188 bp) alleles were analyzed on an agarose gel. In addition, PCR products of deletion (177 bp) and wild-type (174 bp) alleles were analyzed on an agarose gel. BIM polymorphism was analyzed by PCR analysis of peripheral blood (cell-free DNA). DNA extracted from blood samples was diluted with a lysis solution to dissolve erythrocytes. Leukocyte fractions were pelleted and washed once with phosphate-buffered saline, and DNA was extracted from leukocyte pellets by using a QIAamp DNA mini kit (Qiagen KK, Tokyo, Japan).
Measurement of level of GSSG in whole blood and urinary 8-OHdG
A blood sample was collected in a 9 mL tube containing the anticoagulant EDTA. Urine samples were centrifuged at 3,000 rpm for 10 min and stored frozen at −80 °C until analysis. tGSH (i.e., GSH plus GSSG) and GSSG were analyzed with a Bioxytech GSH/GSSG-412 assay kit (Oxis Health Products, Portland, OR, USA). This method uses 5.5'-dithiobis-2-nitrobenzoic acid (DTNB), which reacts with GSH to form a spectrophotometrically detectable product at 412 nm. GSSG is recycled to GSH by glutathione reductase, which requires nicotinamide adenine dinucleotide phosphate (NADPH). This assay kit uses the thiol removal reagent 1-methyl-2-vinylpyridinium trifluoromethanesulfonate (M2VP) at a concentration that rapidly removes GSH but does not interfere with the glutathione reductase assay. The ratio was calculated by using competitive ELISA (New 8-OHdG Check; Nihon Shizuoka Aging Laboratories, Japan) as follows: (GSH ‒ 2 × GSSG)/GSSG (20). The specificity of the monoclonal antibody N45.1 used in the competition ELISA (New 8-OHdG Check; Nihon Shizuoka Aging Laboratories) was previously reported (21). Urinary 8-OHdG is expressed as the ratio of 8-OHdG to creatinine in urine (21).
Pulmonary function testing
Spirometry measurement and carbon monoxide diffusing capacity (DLco) were measured with a pulmonary function test system (Chestac-33, Chest Co. Ltd., Toyo, Japan). Diffusion capacity was measured by the single brace method. % forced vital capacity (FVC) was calculated with the Baldwin formula (22), and %DLco was calculated with the Burrows formula (23). All pulmonary function tests were conducted by two technicians using the methods and standards detailed by the American Thoracic Society (24).
Clinical outcomes
Overall survival (OS), defined as the period from the date of IPF diagnosis until death from any cause, was compared in patients with and without BIM deletion polymorphism. FVC was measured in all patients at baseline and at 1 and 2 years after treatment. In analyses of mean change in forced vital capacity from baseline (ΔFVC), the principle of last observation carried forward was used to address the issue of missing values. We assigned the worst possible outcome to a missing value attributable to patient death (e.g., FVC =0). In addition, missing values due to disease progression outcomes other than death were assigned half the previous value (25).
Statistical analysis
SPSS software for Windows, version 12.0 (SPSS Inc., Tokyo, Japan), was used for the statistical analyses. The clinical characteristics of patients with and without BIM deletion polymorphism were compared with Fisher's exact test. Survival curves were plotted by using the Kaplan–Meier method, and statistical analysis was performed with the log-rank test.
Results
Detection of BIM gene deletion polymorphism in healthy volunteers
Using PCR analysis, we analyzed 30 DNA samples from healthy Japanese volunteers. BIM deletion polymorphism was present in 6 of these 30 (20%) DNA samples.
Detection of BIM gene deletion polymorphism on IPF
BIM deletion polymorphism was present in 8 of the 63 (12.7%) IPF patients analyzed. Clinical characteristics, frequency of IPF treatment [such as N-acetylcysteine (NAC), prednisolone, prednisolone, and cyclosporine (CsA)], levels of serum markers, and pulmonary function test results at baseline did not significantly differ between patients with (n=8) and without (n=55) BIM deletion polymorphism (Table 1). To diagnose IPF, surgical lung biopsy (SLB) was performed in 6 cases (9.5%).
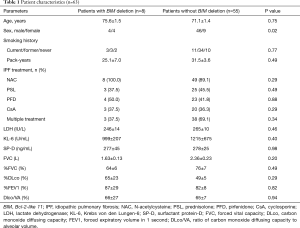
Full table
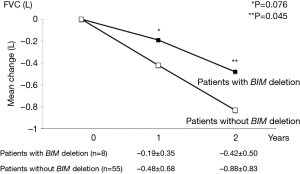
ΔFVC
FVC was measured in all patients at baseline and at 1 and 2 years after treatment. At 1 year, mean ΔFVC was lower in patients with BIM deletion polymorphism than in those without the polymorphism (‒0.19±0.35 vs. ‒0.48±0.68 L, respectively; P=0.076). At 2 years, mean ΔFVC was significantly lower in patients with BIM deletion polymorphism than in those without the polymorphism (‒0.42±0.50 vs. ‒0.88±0.83 L, respectively; P=0.045) (Figure 1).
AE-IPF
We evaluated incidences of AE-IPF and fatal AE-IPF. AE-IPF incidence was significantly lower in patients with BIM deletion polymorphism than in those without the polymorphism (12.5% vs. 54.5%, respectively; P=0.026). The incidence of fatal AE-IPF was significantly lower in patients with BIM deletion polymorphism than in those without the polymorphism (0% vs. 36.4%, respectively; P=0.038) (Table 2).

Full table
Redox markers
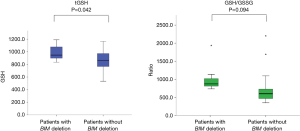
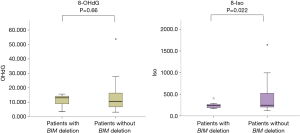
We evaluated baseline redox markers before IPF treatment. GSH/GSSG ratio was higher in patients with BIM deletion polymorphism than in those without the polymorphism (825±158.7 vs. 628.4±41.3, respectively; P=0.094). tGSH was significantly higher in patients with BIM deletion polymorphism (988±48.3 vs. 858±25.8 µM; P=0.042; Figure 2), while 8-iso was significantly lower (246.1±81.6 vs. 400.9±334.1 pg/mL; P=0.022) (Figure 3).
Clinical outcomes
Median OS after diagnosis was longer in patients with BIM deletion polymorphism than in those without the polymorphism (not reached vs. 1,620 days, respectively; P=0.18), but the difference was not significant.
Discussion
BIM deletion polymorphism was observed in 12.7% of Japanese patients with IPF and 20% (6 of 30) of healthy Japanese volunteers; the difference was not statistically significant. The present IPF patients with and without BIM deletion polymorphism did not significantly differ in relation to serologic markers [including lactate dehydrogenase (LDH), Krebs von den Lungen-6 (KL-6), and surfactant protein-D (SP-D)] or pulmonary function test results [including FVC, %FVC, %DLco, % forced expiratory volume in 1 s (FEV1), and ratio of DLco to alveolar volume (DLco/VA)] at baseline. These results suggest that clinical and laboratory test results are insufficient for identifying patients with BIM polymorphism.
Zappala et al. (26) reported that a significant decrease in FVC (>10%) within 6 months is associated with poor prognosis in IPF patients. In this study, mean ΔFVC at 2 years was significantly lower in patients with BIM deletion polymorphism than in those without the polymorphism (–0.42±0.50 vs. –0.88±0.83 L, respectively; P=0.045). AE-IPF is a frequently fatal condition in which rapid pulmonary respiratory failure progresses. The risk of AE-IPF development was reported to be high in IPF patients with decreased FVC (2,27). AE-IPF is identified by the appearance of new ground glass shadows in both lung fields during an otherwise chronic course. IPF is the most frequent cause of death among affected persons (28). The incidences of AE-IPF and fatal AE-IPF were significant lower in patients with BIM deletion polymorphism than in those without the polymorphism [12.5% vs. 54.5% (P=0.026) and 0% vs. 36.4% (P=0.038), respectively]. Median OS after diagnosis was longer in patients with BIM deletion polymorphism than in those without the polymorphism (not reached vs. 1,620 days, respectively; P=0.18). The BIM deletion polymorphism may suppress production of ROS, thus reducing the risks of AE-IPF and fatal AE-IPF. Future studies should attempt to further clarify the effects of BIM deletion polymorphism on AE-IPF.
Interestingly, serologic markers and pulmonary function test results did not differ in relation to BIM deletion polymorphism status; however, baseline redox status, including tGSH and 8-iso, significantly differed between patients with and without this polymorphism. Patients with IPF were reported to have diminished GSH levels in epithelial lining fluid and bronchoalveolar lavage (BAL) fluid and BAL cells (4-7). 8-iso is a nonenzymatic eicosanoid synthesized by random oxidation of tissue phospholipids by active oxygen and was reported to be an excellent indicator of oxidative damage in a rat model of oxidative stress (29). In this study, tGSH at baseline was significantly higher in patients with BIM deletion polymorphism than in those without the polymorphism. A higher GSH/GSSG ratio is associated with a reduction in FVC decline, which could lower the risk of AE-IPF.
The concentration of glutathione, a low molecular weight antioxidant found in normal lung tissue, is diminished in epithelial lining fluid and fibrotic foci of IPF lungs (4,11). The antifibrotic action of the GSH precursor NAC causes NAC to increase lung GSH levels and attenuate bleomycin-induced fibrosis (30,31). Administration of inhaled NAC to IPF patients enhances antioxidant/oxidant balance (32). The PANTHER trial showed that orally administered NAC monotherapy was not effective (33), and the efficacy of NAC inhalation is thus still debated. Future studies should attempt to evaluate the different methods of NAC delivery. In this study, all six IPF patients with BIM deletion polymorphism were treated with inhaled NAC treatment. These patients had decreases in AE and FVC reduction rate, but the benefit of inhaled NAC therapy is unclear for the subset of IPF patients with BIM deletion polymorphism.
IPF is believed to include ROS, particularly lung damage induced by superoxide anion (4-12). Superoxide dismutase (SOD) catalyzes the conversion of superoxide anion to hydrogen peroxide (11,34), and findings from animal studies suggest that lecithinized SOD (PC-SOD) is effective for IPF treatment (35). Clinical trials of PC-SOD for IPF patients are ongoing. Continuing research on therapeutic approaches to inhibit the ROS-mediated response in the development and progression of pulmonary fibrosis show promise for the treatment of IPF.
In the PANTHER trial, Oldham et al. (36) reported SNPs in TOLLIP and MUC5B. NAC therapy was associated with a significant reduction in the risk for the combined endpoint (hazard ratio 0.14; 95% CI, 0.02–0.83; P=0.03) in patients with the TT genotype. The authors concluded that NAC therapy was effective for IPF patients with the TT genotype. Genetic background should be determined in order to predict the clinical effects of NAC treatment, and future clinical trials should use Genetic background as a stratification factor.
A limitation of this study is that it was a retrospective single-center study of a small Japanese population. Second, no patient received nintedanib, and AE-IPF treatment was not standardized. Inhaled NAC improved redox imbalance in a previous study of 22 IPF patients (37). A large-scale multicenter study with a validation cohort is necessary in order to confirm the present results. Third, we reported a difference in FVC progression in the presence or absence of BIM gene polymorphism but could not comprehensively evaluate the effect of IPF treatment.
The effectiveness of NAC treatment should be evaluated in patients stratified by BIM deletion polymorphism status. We assigned the worst possible outcome to a missing value attributable to patient death; values missing because of disease progression outcomes other than death were assigned half the previous value. Whether this is a substitute for lung function needs to be examined in a future study.
Conclusions
In conclusion, the present results suggest that clinical outcomes are better for IPF patients with BIM deletion polymorphism than for those without the polymorphism, perhaps because of differences in redox status.
Acknowledgments
We thank Atsushi Kakimoto of Konica Minolta Inc. (Tokyo, Japan), Nobuyuki Shiraga of the Department of Diagnostic Radiology, Toho University Omori Medical Center (Tokyo, Japan), and Atsuko Kurosaki of the Department of Diagnostic Radiology, Fukujuji Hospital (Tokyo, Japan). We are also grateful to David Kipler for his review of the language of this article.
Funding: This study was partially supported by a grant from the Ministry of Health, Labour and Welfare of Japan, awarded to the Study Group on Diffuse Pulmonary Disorders, Scientific Research/Research on Intractable Disease, by JSPS KAKENHI Grant Numbers JP15K09195 and JP2642140, and by a Research Promotion Grant from Toho University Graduate School of Medicine (No. 16-3, to K.I.).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Institutional Review Board of Toho University Medical Center Omori Hospital (25-5).
References
- Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016;194:265-75. [Crossref] [PubMed]
- Song JW, Hong SB, Lim CM, et al. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J 2011;37:356-63. [Crossref] [PubMed]
- Kondoh Y, Taniguchi H, Ebina M, et al. Risk factors for acute exacerbation of idiopathic pulmonary fibrosis--Extended analysis of pirfenidone trial in Japan. Respir Investig 2015;53:271-8. [Crossref] [PubMed]
- Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis 1989;139:370-2. [Crossref] [PubMed]
- Meyer A, Buhl R, Magnussen H. The effect of oral N-acetylcysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J 1994;7:431-6. [Crossref] [PubMed]
- Behr J, Degenkolb B, Maier K, et al. Increased oxidation of extracellular glutathione by bronchoalveolar inflammatory cells in diffuse fibrosing alveolitis. Eur Respir J 1995;8:1286-92. [Crossref] [PubMed]
- Behr J, Degenkolb B, Krombach F, et al. Intracellular glutathione and bronchoalveolar cells in fibrosing alveolitis: effects of N-acetylcysteine. Eur Respir J 2002;19:906-11. [Crossref] [PubMed]
- Gross TJ, Hunninghake GW. Idiopathic pulmonary fibrosis. N Engl J Med 2001;345:517-25. [Crossref] [PubMed]
- Bargagli E, Olivieri C, Bennett D, et al. Oxidative stress in the pathogenesis of diffuse lung diseases: a review. Respir Med 2009;103:1245-56. [Crossref] [PubMed]
- Kondrikov D, Caldwell RB, Dong Z, et al. Reactive oxygen species-dependent RhoA activation mediates collagen synthesis in hyperoxic lung fibrosis. Free Radic Biol Med 2011;50:1689-98. [Crossref] [PubMed]
- Kliment CR, Oury TD. Oxidative stress, extracellular matrix targets, and idiopathic pulmonary fibrosis. Free Radic Biol Med 2010;49:707-17. [Crossref] [PubMed]
- Manoury B, Nenan S, Leclerc O, et al. The absence of reactive oxygen species production protects mice against bleomycin-induced pulmonary fibrosis. Respir Res 2005;6:11. [Crossref] [PubMed]
- Kuwano K, Miyazaki H, Hagimoto N, et al. The involvement of Fas-Fas ligand pathway in fibrosing lung diseases. Am J Respir Cell Mol Biol 1999;20:53-60. [Crossref] [PubMed]
- Gong Y, Somwar R, Politi K, et al. Induction of BIM is essential for apoptosis triggered by EGFR kinase inhibitors in mutant EGFR-dependent lung adenocarcinomas. PLoS Med 2007;4:e294. [Crossref] [PubMed]
- Faber AC, Corcoran RB, Ebi H, et al. BIM expression in treatment-naive cancers predicts responsiveness to kinase inhibitors. Cancer Discov 2011;1:352-65. [Crossref] [PubMed]
- Hagenbuchner J, Kuznetsov A, Hermann M, et al. FOXO3-induced reactive oxygen species are regulated by BCL2L11 (Bim) and SESN3. J Cell Sci 2012;125:1191-203. [Crossref] [PubMed]
- Ng KP, Hillmer AM, Chuah CT, et al. A common BIM deletion polymorphism mediates intrinsic resistance and inferior responses to tyrosine kinase inhibitors in cancer. Nat Med 2012;18:521-8. [Crossref] [PubMed]
- Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018;198:e44-68. [Crossref] [PubMed]
- Homma S, Bando M, Azuma A, et al. Japanese guideline for the treatment of idiopathic pulmonary fibrosis. Respir Investig 2018;56:268-91. [Crossref] [PubMed]
- Nijmeh J, Moldobaeva A, Wagner EM. Role of ROS in ischemia-induced lung angiogenesis. Am J Physiol Lung Cell Mol Physiol 2010;299:L535-41. [Crossref] [PubMed]
- Shimoike T, Inoguchi T, Umeda F, et al. The meaning of serum levels of advanced glycosylation end products in diabetic nephropathy. Metabolism 2000;49:1030-5. [Crossref] [PubMed]
- Baldwin ED, Cournand A, Richards DW Jr. Pulmonary insufficiency; physiological classification, clinical methods of analysis, standard values in normal subjects. Medicine (Baltimore) 1948;27:243-78. [Crossref] [PubMed]
- Burrows B, Kasik JE, Niden AH, et al. Clinical usefulness of the single-breath pulmonucy diffusing capacity test. Am Rev Respir Dis 1961;84:789-806. [PubMed]
- Standardization of Spirometry. 1994 Update. American Thoracic Society. Am J Respir Crit Care Med 1995;152:1107-36. [Crossref] [PubMed]
- King TE Jr, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2083-92. [Crossref] [PubMed]
- Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010;35:830-6. [Crossref] [PubMed]
- Costabel U, Inoue Y, Richeldi L, et al. Efficacy of Nintedanib in Idiopathic Pulmonary Fibrosis across Prespecified Subgroups in INPULSIS. Am J Respir Crit Care Med 2016;193:178-85. [Crossref] [PubMed]
- Natsuizaka M, Chiba H, Kuronuma K, et al. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med 2014;190:773-9. [Crossref] [PubMed]
- Hozawa A, Ebihara S, Ohmori K, et al. Increased plasma 8-isoprostane levels in hypertensive subjects: the Tsurugaya Project. Hypertens Res 2004;27:557-61. [Crossref] [PubMed]
- Giri SN, Hyde DM, Schiedt MJ. Effects of repeated administration of N-acetyl-L-cysteine on sulfhydryl levels of different tissues and bleomycin-induced lung fibrosis in hamsters. J Lab Clin Med 1988;111:715-24. [PubMed]
- Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med 2000;162:225-31. [Crossref] [PubMed]
- Borok Z, Buhl R, Grimes GJ, et al. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet 1991;338:215-6. [Crossref] [PubMed]
- Idiopathic Pulmonary Fibrosis Clinical Research Network, Martinez FJ, de Andrade JA, et al. Randomized trial of acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med 2014;370:2093-101. [Crossref] [PubMed]
- Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 2003;167:1600-19. [Crossref] [PubMed]
- Tanaka K, Ishihara T, Azuma A, et al. Therapeutic effect of lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 2010;298:L348-60. [Crossref] [PubMed]
- Oldham JM, Ma SF, Martinez FJ, et al. TOLLIP, MUC5B, and the Response to N-Acetylcysteine among Individuals with Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2015;192:1475-82. [Crossref] [PubMed]
- Muramatsu Y, Sugino K, Ishida F, et al. Effect of inhaled N-acetylcysteine monotherapy on lung function and redox balance in idiopathic pulmonary fibrosis. Respir Investig 2016;54:170-8. [Crossref] [PubMed]


