
Clinical utility of robot-assisted transthoracic esophagectomy in advanced esophageal cancer after neoadjuvant chemoradiation therapy
Introduction
The survival rate of locally-advanced esophageal cancer, defined as ≥ T2 or node-positive disease, is significantly lower than early esophageal cancer, with the 5-year survival rate ranging from 15% to 34% (1). Consequently, multimodality neoadjuvant chemoradiation therapy (nCRT) treatment followed by surgery has become a standard treatment for locally-advanced esophageal cancer in the National Comprehensive Cancer Network (NCCN) guidelines (2).
On the other hand, mortality and morbidity rates of esophageal cancer have declined significantly with the introduction of minimal invasive techniques (3). More recently, robotic assistance systems have been expanding in use and may overcome some of the limitations of thoraco-laparoscopy (4).
Multiple studies have investigated the surgical outcome of robot-assisted minimal invasive thoraco-laparoscopic esophagectomy (RAMIE) (5-8). However, there are far fewer RAMIE cases regarding advanced esophageal cancer after nCRT, and surgical outcomes in these patients remain unclear. Therefore, we examined the surgical and oncological outcomes of RAMIE compared with open esophagectomy (OE) in advanced esophageal cancer patients after nCRT. In addition, we conducted a statistical analysis between the RAMIE patients who did and did not undergo nCRT, to assess the prognostic effects of nCRT on postoperative complications.
Methods
Patients
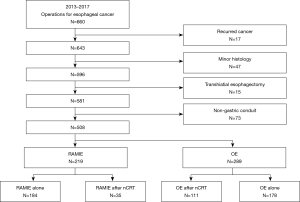
All clinical records of patients who underwent surgery for esophageal cancer were retrospectively collected between January 2013 and December 2017 in the Thoracic Surgery Department at Asan Medical Center, Seoul, South Korea (n=660). Exclusion criteria were as follows: (I) recurrent esophageal cancer (n=17); (II) histology other than squamous cell carcinoma (n=47); (III) transhiatal esophagectomy (n=15), and (IV) non-gastric conduit (n=73) (Figure 1). The remaining 508 patients who received an Ivor Lewis or McKeown procedure were allowed to choose whether to undergo OE or RAMIE. Among them, 219 patients (43.1%) underwent the RAMIE procedure, whereas 289 patients (56.9%) underwent OE. In patients who received the RAMIE procedure, 35 patients (16.0%) underwent nCRT for locally-advanced esophageal cancer (RAMIE after nCRT). On the other hand, among the 289 patients who underwent OE, 111 patients (38.4%) received nCRT preoperatively (OE after nCRT). We also collected information about patients who underwent RAMIE alone without nCRT (n=184) Esophageal resection and reconstruction procedures were performed by a single thoracic surgeon (YH Kim), assisted by the robotic system (da Vinci Si or Xi surgical system, Intuitive Surgical, Sunnyvale, CA, USA). This study was approved by the Asan Medical Center Ethics Committee/Review Board.
nCRT
The pre-operative staging system included endoscopic ultrasound (EUS), chest computed tomography (CT), abdominal CT, pelvic CT, and positron emission tomography (PET) scanning as per NCCN guidelines (2). If a lymph node metastasis was suspected by imaging modalities, EUS or endobronchial ultrasound (EBUS)-guided fine needle aspiration followed to confirm findings. After staging, patients who had locally-advanced disease (≥ T2 or ≥ N1) received nCRT, except when the patient was over 75 years of age or in poor physical condition, according to the oncologist’s judgment from a multidisciplinary oncologic clinic. Patients treated at our institution received oxaliplatin and titanium silicate-1, with concurrent external beam radiation, for a total dose of 46 Gy in 23 fractions of 2.0 Gy each over the course of 5–6 weeks (9). Four weeks after concluding therapy, repeat PET-CT scans were performed for restaging. All patients were then offered esophagectomy, except for patients with distant metastasis to major organs (liver, lung, spine, brain, etc.) or who refused surgery.
Contraindication and technique of RAMIE
Patients with bulky tumors or large metastatic lymph nodes attached to the trachea that were difficult to dissect with current robotic devices were contraindicated for the RAMIE procedure. Additionally, we excluded patients with highly suspected tracheal invasion of tumors and lymph nodes in preoperative imaging modalities. However, if remarkable remission and a clear boundary with adjacent structures were shown in the imaging work-up (gastric fibroscopy, CT, and PET scan) reevaluation one month after nCRT, we included them as a candidate for RAMIE.
The RAMIE procedures were comprised of conduit formation (laparoscopic or robotic) and robot-assisted thoracic esophagectomy. Gastric conduit formation was conducted by an experienced stomach surgeon with a laparoscope in the supine position. Five laparoscopic ports were placed for the abdominal procedure. The conduit was made within the abdomen, but was left partially connected to the proximal stomach for easy retrieval from the chest. The left gastric, celiac, gastrohepatic, paracardial, and diaphragm lymph node dissections were completed during the abdominal phase. The patient was placed in a left-sided semi-prone position for the sequential thoracic procedures. Three 8-mm ports were placed to dock the robot system and one 12-mm port was used by the first assistant. Carbon dioxide (CO2) gas was routinely insufflated at 4 to 6 mmHg into the thorax. In case of the Ivor Lewis procedure, an 8-mm sized camera port in the 5th intercostal space was extended up to 3-cm to take out the resected esophagogastric specimen from the thorax and then place the circular stapler into the thorax. After partial resection of the esophagus at an anastomosis level, a manual purse-string suture using a robotic arm (Maryland forceps or needle holder) was placed at the highest level of the thoracic esophagus. The anvil part of an endo-circular stapler was inserted in the proximal esophagus through the esophageal opening, and the purse-string suture was tied around the central rod. The dissected esophagus and remnant stomach were then removed. As in the conventional Ivor Lewis procedure, the anastomosis was always performed above the azygos vein. Intra-operative frozen sectioning was used to confirm negative margins. The left, right recurrent laryngeal, subcarinal, hilar, azygous vein, upper, middle, lower para-esophageal, and inferior pulmonary ligament lymph nodes were routinely resected during the thoracic phase. The upper and lower paratracheal as well as interlobar lymph nodes were harvested in patients with preoperatively suspected or confirmed lymph node invasion-metastases, or who underwent nCRT due to multi-station lymph node metastasis. For an esophagogastric anastomosis in Ivor Lewis or McKeown procedure, circular staplers were mostly utilized and a manual suture was rarely performed in patients with a relatively short or tight gastric conduit, which were either hand- or robot-assisted. The decision whether to perform the Ivor Lewis or McKeown procedure was determined depending on the relative position of the tumor and the carina. If the upper margin of the tumor was below the carina, Ivor Lewis procedure was usually performed, otherwise McKeown procedure was done. If lymph node metastasis was highly suspected in upper esophageal area, McKeown procedure was preferred, regardless of the tumor location. If the length of the conduit was not appropriate, Ivor Lewis was performed on the premise that the resection margin could be secured. Cervical node dissection was performed by a head and neck surgeon if the patient had cervical esophageal cancer, advanced T stage upper thoracic esophageal cancer, or a suspected cervical/highest mediastinal lymph node metastasis before nCRT.
Post-operative care
Patients were usually extubated in the operating room and admitted to the intensive care unit (ICU) overnight for observation. The nasogastric tube was routinely removed one day after surgery. On postoperative day 4, an esophagography was performed to find leaks and delayed gastric emptying. If the esophagography was normal, the patient started on a clear liquid diet. The chest tube was removed once patients tolerated a soft diet and there was no evidence of chylothorax, empyema, air leakage, and excessive tube drainage (>3 mL/kg/day).
If patients showed hoarseness after the operation, hyaluronic acid injection into vocal fold was performed in patients with unilateral vocal cord injury. In case of bilateral injury, patients were carefully observed if the airway was patent, otherwise tracheostomy was performed. We underwent jejunostomy insertion in most patients with bilateral vocal cord injury for the concern of severe aspiration and the lack of nutritional supply.
Clinical follow-up data were obtained from our outpatient clinic and patient deaths were captured by record review from the Korean National Security Death Index. Patients were staged according to the American Joint Committee on Cancer (AJCC) 8th edition TNM staging definitions (10).
Definitions
The primary outcomes of interest were major postoperative complications (i.e., anastomotic leakage, postoperative bleeding requiring reoperation, bronchopleural fistula (BPF), chyloperitoneum, chylothorax requiring duct ligation or radiologic embolization, conduit necrosis, superficial wound problems requiring reoperation, vocal cord injury, all-cause reoperation, pneumonia, acute respiratory distress syndrome, prolonged ventilation (≥24 hours), arrhythmia, vasopressors use, or cerebrovascular events. The secondary outcomes of interest were early mortality (in-hospital, 30-day, 90-day, 1-year), early recurrence within 1 year (i.e., locoregional, distant, and mixed).
Almost all major complications were evaluated according to the Society of Thoracic Surgeons and the European Society of Thoracic Surgeons joint definitions (11). The complication of anastomotic leakage was defined according to the Esophageal Complication Consensus Group guidelines (ECCG) (12) and the severity of complications was graded using the Clavien-Dindo classification (13).
Statistical analysis
Comparisons of patient characteristics among the two treatment groups were performed using the χ2 test for categorical variables (frequency and percentages) and the two-sample unpaired t-test for continuous variables (mean and standard deviations). If expected frequencies were <5, a two-tailed Fisher’s exact probability test was used. The impact of RAMIE or nCRT on early clinical outcomes was evaluated using univariate analysis of logistic regression.
All statistical calculations were performed using R version 3.2.5 (The R Foundation for Statistical Computing, Vanderbilt University, Nashville, TN, USA) using the Survival, GGally, ggplot2 and forestplot packages. The P values less than 0.05 were considered significant.
Results
Comparison of clinical information
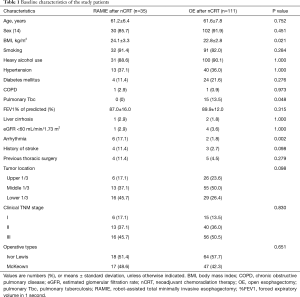
The baseline characteristics of the patients in the study are described in Table 1. The median duration of follow-up was 21.2 months (range, 2.0–63.0 months) in the RAMIE after nCRT group and 26.5 months (range, 2.0–64.0 months) in the OE after nCRT group.
Full table
Between the RAMIE and the OE patients after nCRT, there were no significant differences in age, sex, medical history such as smoking, heavy alcohol use, hypertension, diabetes mellitus, chronic obstructive pulmonary disease and history of thoracic surgery. The OE after nCRT group had more patients with a history of pulmonary tuberculosis, whereas the RAMIE group revealed a higher body mass index and history of arrhythmia. There were no intergroup differences in the distribution of tumor location and clinical TNM stages, and the ratio of McKeown to Ivor Lewis operations was also not significantly different between the two groups.
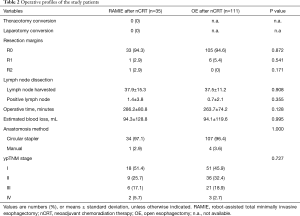
Table 2 presents the operative profiles of the two groups. R0 resection was achieved similarly in 94.3% and 94.6% of the patients in the RAMIE after nCRT group and the OE after nCRT group, respectively (P=0.872). The mean numbers of harvested lymph nodes and metastatic lymph nodes were not significantly different between the two groups (P=0.908 and 0.355). The mean operation time appeared longer in the RAMIE after nCRT group (286.2±80.8 minutes) than the OE after nCRT group (263.7±74.2 minutes) without statistical significance (P=0.128). There were no significant differences in estimated blood loss, anastomosis method and ypTNM stage between the RAMIE and OE patients after nCRT.
Full table
Postoperative clinical outcomes of patients are depicted in Table 3. There was one case of in-hospital death only in the OE after nCRT group (0.9%, n=1/111); this patient underwent surgical drainage of the mediastinal abscess due to a BPF and died of septic shock and multi-organ failure on postoperative day 88. The mortality rate within 1 year was 20.0% in the RAMIE after nCRT group and 25.2% in the OE after nCRT group, and there were no significant intergroup differences (P=0.686). In terms of vocal cord injury, 2 patients were in Ivor Lewis (25.0%) procedure and 6 patients were in McKeown procedure (75.0%) in RAMIE group (n=8). In OE group (n=28), 5 patients were in Ivor Lewis (17.9%) and 23 patients were in McKeown procedure (82.1%). The causes for reoperation were postoperative bleeding (n=1) and abdominal wound problem (n=1) in RAMIE group and postoperative bleeding (n=1), abdominal wound problem (n=5), anastomotic leakage (n=1), chylothorax (n=1), and BPF (n=1) in OE group. The early recurrence rate within 1 year was not significantly different between the RAMIE after nCRT group and the OE after nCRT group (17.1% vs. 13.5%, P=0.797).

Full table
There were no significant intergroup differences of postoperative morbidity between the two groups, except the higher use of vasopressor (P<0.001) in the OE group. The distribution of severity according to Clavien-Dindo classification was not also significantly different (P=0.926).
Prognostic effect of RAMIE and nCRT on postoperative complications
After defining each postoperative complication as outcome variables, we performed univariate logistic regression with the predictive variables of RAMIE (Figure 2) and nCRT (Figure 3). Some complications, such as BPF and conduit necrosis, were too rare to perform logistic regression.
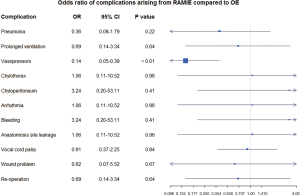
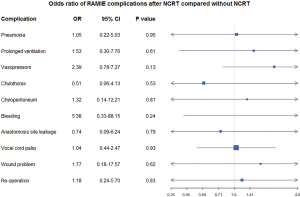
For patients after nCRT, the RAMIE procedure was found to be associated with a significant risk reduction for the use of vasopressors [odds ratio (OR) 0.14, 95% CI: 0.05–0.39; P<0.01]. There were no significant inter-group differences in other outcome variables, including pneumonia, chylothorax, chyloperitoneum, arrhythmia, postoperative bleeding, anastomosis leakage, vocal cord palsy, wound problem, and re-operation (Figure 2). Among all patients who underwent RAMIE in this study, there was no definite evidence of the negative prognostic effect of nCRT in all postoperative complications (Figure 3).
Discussion
Since the robotic system first appeared as a treatment for esophageal cancer in 2000 (15), the number of RAMIE procedures has been steadily rising universally, with more than 1,800 cases being performed in 2016 (7). The oncological long-term outcome of RAMIE has also been reported, indicating effective and acceptable overall survival and recurrence rates (16,17). Recently, a randomized controlled ROBOT trial was reported to compare robot-assisted and open three-phase esophagectomy (18). According to the ROBOT trial, the use of RAMIE showed superior postoperative outcomes (including overall, surgery-related, and cardiopulmonary complications) to OE in patients with resectable esophageal cancer, regardless of their clinical stage. However, due to concerns about feasibility, safety, and oncological outcomes, as well as a demanding learning curve, patients who received nCRT are generally regarded as ineligible for robotic surgery. Therefore, in the present study, we evaluated the incidence of postoperative complication and early outcomes by selecting patients with locally-advanced esophageal cancer who underwent nCRT.
With similar preoperative findings and distributions of clinical TNM and ypTNM stages, the RAMIE after nCRT group did not show a significant increase in operative time or estimated blood loss compared to the OE after nCRT group. There were no early deaths in the RAMIE group, whereas one patient died 88 days after surgery in the OE group. In addition to similar high R0 rates (94.3% vs. 94.6%, P=0.872), incidences of post-operative surgical complications including anastomotic leakage, arrhythmia, wound infection, chylothorax, vocal cord palsy and re-operation did not differ between the two groups. In terms of pulmonary complication, we found a lower incidence of pneumonia in the RAMIE group (2/35, 5.7%) than the OE group (15/111, 13.5%), where the statistical significance was not shown (P=0.341). With regard to cardiac complications, the use of vasopressors was significantly lower in RAMIE patients and also confirmed as a negative prognostic factor by logistic univariate analysis (HR =0.14, P<0.01).
The inverse relationship between postoperative use of vasopressor and the RAMIE procedure has not previously been described. This should be interpreted with caution because there may have been unexpected confounding factors, considering the limitation of univariate analysis. However, after correction with several covariates (such as age, sex, past medical history, clinical TNM and ypTNM stages) on multivariate logistic regression (not shown), the inverse association remained statistically significant (HR =0.13, P<0.001). This phenomenon could be explained by the difference of the postoperative inflammatory response between RAMIE and OE groups. Specifically, the response of postoperative C-reactive protein, interleukin-6, interleukin-8, and production of reactive oxygen species in neutrophils was weakened in the minimally invasive approach as compared with open thoracotomy (19,20). Based on these findings, it can be inferred that OE patients are more likely to suffer hypovolemia and resultant hypotension from reactive systemic vasodilation, impaired vascular permeability, and third space loss. Consequently, postoperative hypotension might not always be treated by fluid replacement alone, but also by inotropic agents occasionally to avoid excessive hydration in OE patients.
With regard to lymph node dissection, we underwent nearly 40 lymph node harvesting procedures in patients with RAMIE after nCRT and OE after nCRT, which are markedly higher, compared to other studies (18 to 30 lymph nodes, on average) (5,21). As a result of these aggressive lymphadenectomy procedures, higher rates of vocal cord palsy were found in RAMIE (22.9%) and OE group (25.2%) in the present study as compared to other studies (4% to 19%) (21). However, the rate of permanent vocal fold paralysis after an early period decreased drastically to 5% and 7% in the RAMIE and the OE after nCRT patients, respectively. This consequence is also described in other studies, in which 26% and 29% of vocal cord palsy occurred after 44 and 38 of lymph node harvesting procedures (22,23).
It should be mentioned that all RAMIEs in our study were conducted under a collaborative system with specialized teams, including stomach, thoracic, and neck surgeons, in a state beyond the learning curve. This might be the main reason the RAMIE group revealed comparable operative time and postoperative outcome with the OE group in advanced-stage patients after nCRT. Additionally, the prognostic impact of nCRT was shown to be statistically insignificant between the RAMIE patients who received nCRT and those who did not.
There are several limitations in the current study: (I) selection bias is inherent in retrospective analysis as patients with cervical esophageal cancer and advanced T stage cancer invading the trachea preferred conventional OE to achieve R0 resection; (II) all RAMIE procedures were conducted by a single surgeon from a single center; (III) a relatively small number of patients were enrolled in the current study, thereby several outcome variables such as pneumonia, which appeared to differ between RAMIE and OE (5.6% vs. 14.1%), were not statistically significant (P=0.293). We also had to use patients with transthoracic approach that consisted of two different surgical procedures (Ivor Lewis and McKeown procedure) as a group in our study. However, there were no significant differences in major complications other than vocal cord palsy between Ivor Lewis and McKeown procedure in our patients. In addition, the distribution of IL and 3-hole patients in our study was similar in RAMIE and OE group; and (IV) Long-term outcomes associated with survival, recurrence, complications, and quality of life could not be analyzed due to our short-term follow-up, which is important to determine the best surgical option for advanced esophageal cancer. A prospective, long-term follow-up, multi-center randomized controlled study is needed to overcome our limitations.
Conclusions
In patients who received nCRT for locally-advanced esophageal cancer, RAMIE is a safe and feasible treatment with a comparable early mortality, early recurrence rate, and postoperative morbidity, as compared to OE. Furthermore, administration of nCRT did not significantly affect the postoperative morbidity among patients who underwent RAMIE. With surgeons who have adequate experience and careful surgical planning, adopting RAMIE would be an appropriate option for patients with advanced stage esophageal cancer after nCRT.
Acknowledgments
The authors would like to thank all stomach surgeons for their dedication and invaluable help to the research.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the institutional review board of Asan Medical Center in Seoul, South Korea (IRB No. 2016-0378) and the requirement for individual patient consent was waived.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241-52. [Crossref] [PubMed]
- Strong VE, D'Amico TA, Kleinberg L, et al. Impact of the 7th Edition AJCC staging classification on the NCCN clinical practice guidelines in oncology for gastric and esophageal cancers. J Natl Compr Canc Netw 2013;11:60-6.
- Biere SS, van Berge Henegouwen MI, Maas KW, et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet 2012;379:1887-92. [Crossref] [PubMed]
- Shridhar R, Abbott AM, Doepker M, et al. Perioperative outcomes associated with robotic Ivor Lewis esophagectomy in patient's undergoing neoadjuvant chemoradiotherapy. J Gastrointest Oncol 2016;7:206-12. [PubMed]
- Cerfolio RJ, Wei B, Hawn MT, et al. Robotic Esophagectomy for Cancer: Early Results and Lessons Learned. Semin Thorac Cardiovasc Surg 2016;28:160-9. [Crossref] [PubMed]
- Ruurda JP, van der Sluis PC, van der Horst S, et al. Robot-assisted minimally invasive esophagectomy for esophageal cancer: A systematic review. J Surg Oncol 2015;112:257-65. [Crossref] [PubMed]
- Seto Y, Mori K, Aikou S. Robotic surgery for esophageal cancer: Merits and demerits. Ann Gastroenterol Surg 2017;1:193-8. [Crossref] [PubMed]
- Weksler B, Sullivan JL. Survival After Esophagectomy: A Propensity-Matched Study of Different Surgical Approaches. Ann Thorac Surg 2017;104:1138-46. [Crossref] [PubMed]
- Yoon DH, Jang G, Kim JH, et al. Randomized phase 2 trial of S1 and oxaliplatin-based chemoradiotherapy with or without induction chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2015;91:489-96. [Crossref] [PubMed]
- Brierley JD, Gospodarowicz MK, Wittekind C. editors. TNM classification of malignant tumours. 8th edition. Chichester, West Sussex, UK; Hoboken, NJ: John Wiley & Sons, Inc., 2017.
- Fernandez FG, Falcoz PE, Kozower BD, et al. The Society of Thoracic Surgeons and the European Society of Thoracic Surgeons general thoracic surgery databases: joint standardization of variable definitions and terminology. Ann Thorac Surg 2015;99:368-76. [Crossref] [PubMed]
- Low DE, Alderson D, Cecconello I, et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann Surg 2015;262:286-94. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Al-Batran SE, Hofheinz RD, Pauligk C, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol 2016;17:1697-708. [Crossref] [PubMed]
- Hashizume M, Shimada M, Tomikawa M, et al. Early experiences of endoscopic procedures in general surgery assisted by a computer-enhanced surgical system. Surg Endosc 2002;16:1187-91. [Crossref] [PubMed]
- Park SY, Kim DJ, Do YW, et al. The Oncologic Outcome of Esophageal Squamous Cell Carcinoma Patients After Robot-Assisted Thoracoscopic Esophagectomy With Total Mediastinal Lymphadenectomy. Ann Thorac Surg 2017;103:1151-7. [Crossref] [PubMed]
- van der Sluis PC, Ruurda JP, Verhage RJ, et al. Oncologic Long-Term Results of Robot-Assisted Minimally Invasive Thoraco-Laparoscopic Esophagectomy with Two-Field Lymphadenectomy for Esophageal Cancer. Ann Surg Oncol 2015;22 Suppl 3:S1350-6. [Crossref] [PubMed]
- van der Sluis PC, van der Horst S, May AM, et al. Robot-assisted Minimally Invasive Thoracolaparoscopic Esophagectomy Versus Open Transthoracic Esophagectomy for Resectable Esophageal Cancer: A Randomized Controlled Trial. Ann Surg 2019;269:621-30. [Crossref] [PubMed]
- Craig SR, Leaver HA, Yap PL, et al. Acute phase responses following minimal access and conventional thoracic surgery. Eur J Cardiothorac Surg 2001;20:455-63. [Crossref] [PubMed]
- Nagahiro I, Andou A, Aoe M, et al. Pulmonary function, postoperative pain, and serum cytokine level after lobectomy: a comparison of VATS and conventional procedure. Ann Thorac Surg 2001;72:362-5. [Crossref] [PubMed]
- Sarkaria IS, Rizk NP, Finley DJ, et al. Combined thoracoscopic and laparoscopic robotic-assisted minimally invasive esophagectomy using a four-arm platform: experience, technique and cautions during early procedure development. Eur J Cardiothorac Surg 2013;43:e107-15. [Crossref] [PubMed]
- Park SY, Kim DJ, Yu WS, et al. Robot-assisted thoracoscopic esophagectomy with extensive mediastinal lymphadenectomy: experience with 114 consecutive patients with intrathoracic esophageal cancer. Dis Esophagus 2016;29:326-32. [Crossref] [PubMed]
- Kim DJ, Hyung WJ, Lee CY, et al. Thoracoscopic esophagectomy for esophageal cancer: feasibility and safety of robotic assistance in the prone position. J Thorac Cardiovasc Surg 2010;139:53-59.e1. [Crossref] [PubMed]


