A new technology for reducing anastomotic fistula in the neck after esophageal cancer surgery
Introduction
Cervical esophagogastric anastomosis and thoracic esophagogastric anastomosis are the two most commonly used surgical methods for esophageal cancer (1,2). The two anastomosis methods have their own advantages and disadvantages. The procedure of cervical esophagogastric anastomosis is simple, and the required traditional surgical skills are low; thus, this technique is more popular among young doctors. However, cervical anastomosis is more likely to cause stenosis and fistula than intrathoracic anastomosis (3). Data show that the incidence of cervical anastomotic stenosis is approximately 15–35% (4,5), the incidence of fistula is approximately 5–10% (6,7), and the incidence of thoracic anastomotic mediastinal fistula is approximately 3–5% (8). The use of a mechanical stapler in the neck reduces postoperative stenosis caused by manual operation to a certain extent, but esophageal stenosis is a composite result of multiple factors. The use of a single staple plays a limited role in reducing anastomotic stenosis, and cervical anastomotic fistula is also a comprehensive result of multiple factors (9). Based on the use of a mechanical stapler, in this study, we creatively employed Neoveil® (10) during embedding at the esophageal anastomotic orifice to find a new method to reduce anastomotic stenosis and fistula after esophageal cancer surgery. The relevant results are summarized below.
Methods
Study subjects
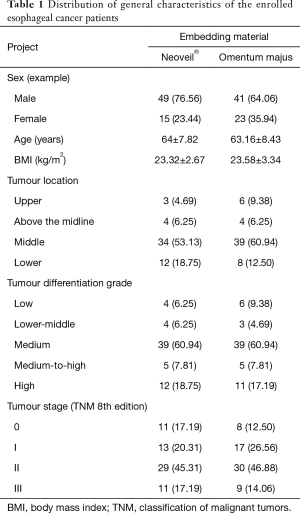
A total of 128 patients with esophageal cancer admitted to the Department of Thoracic Surgery of The First Affiliated Hospital of Zhengzhou University from March 2018 to November 2018 were selected. The inclusion criteria for patients were as follows: undergoing cervical anastomosis with a mechanical stapler; tubular stomach used as the esophageal substitute; survival during the follow-up period; and with complete preoperative and postoperative medical records. The exclusion criteria were as follows: full manual end-side anastomosis; undergoing Sweet or Ivor-Lewis surgery; death during the follow-up period; and missing intraoperative and postoperative case data. There were 90 males and 38 females. There was no significant difference in age, sex, body mass index, blood type, tumour location, tumour differentiation, tumour stage, or other relevant data between the two groups (P>0.05). All patients underwent an enhanced chest CT plus abdominal plain scan before surgery in addition to ultrasound gastroscopy and thoracic duct imaging to determine the lesion site and infiltration level. The patients were screened for metastasis and serious cardiovascular diseases. The patients were divided into two groups according to the embedding materials, and the relative data distribution is shown in Table 1.
Full table
Surgical method
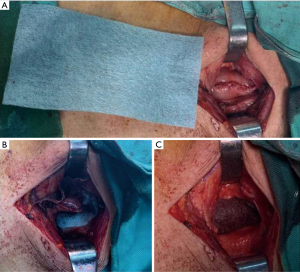
The operation was divided into two stages. In the first stage, the patient was in the left lateral position, and observation windows were established in the 7th rib of the right midaxillary line, the 4th intercostal space of the right axillary front, the 5th intercostal space of the midaxillary line, and the 9th intercostal space of the posterior axillary line. In the second stage, the patient returned to the supine position and raised his left shoulder. An observation window was established 1 cm to the left and below the umbilicus, and another 4 operation windows were established around the abdomen. The lower esophagus and stomach were dissociated according to routine methods, and the tubular stomach was isolated. The pruned omental membrane was tied to the end of the tubular stomach and pulled out of the abdominal cavity through the thoracic cavity for neck anastomosis. The omentum majus embedding group: Interstitial embedding of the residual gastric muscle layer was performed along the anastomotic site. The omentum majus head was lifted and cut open by 2–3 cm along a circular path, and an anastomotic window was created below the omentum majus to stitch the cut ends together with a suture needle tip to complete embedding. The Neoveil® embedding group: No part of the omentum majus was reserved. After the completion of mechanical neck anastomosis, lamellar Neoveil® repair scissors were wrapped around the anastomotic site, and both ends of Neoveil® were sutured on the plasma muscle layer of the anastomotic site to complete embedding (Figure 1).
Assessment criteria
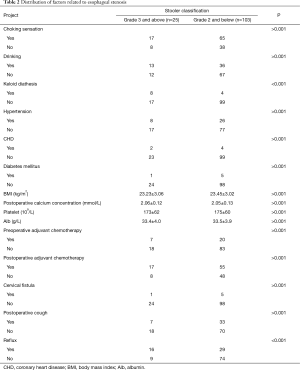
Stenosis was classified into 5 levels according to the Stooler classification: grade 0, anastomotic diameter ≥9 mm, can eat normal food; grade 1, 7 mm ≤ anastomotic diameter <9 mm, obstruction in the consumption of soft food; grade 2, 5 mm ≤ anastomotic diameter <7 mm, can consume a semi-liquid diet; grade 3, 3 mm ≤ anastomotic diameter <5 mm, can consume a liquid diet; and grade 4, anastomotic diameter <3 mm, difficulty or inability in ingesting liquids. In this study, the anastomotic diameter was combined with the patient’s symptoms. Patients with anastomotic stenosis who could not eat solid food and whose symptoms could only be relieved by balloon dilation were included (Table 2).
Full table
Statistical analysis
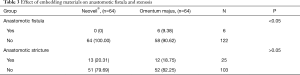
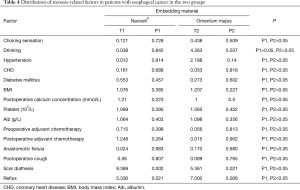
Statistical data for this study were analysed using SPSS 21.0 software. Measurement data are expressed as  . Independent sample t tests were performed for both groups. The relationship between embedding materials and anastomotic fistula and stenosis was evaluated usingχ2 tests (Table 3). P<0.05 was statistically significant. The distribution of anastomotic stenosis in the two groups is shown in Table 4.
. Independent sample t tests were performed for both groups. The relationship between embedding materials and anastomotic fistula and stenosis was evaluated usingχ2 tests (Table 3). P<0.05 was statistically significant. The distribution of anastomotic stenosis in the two groups is shown in Table 4.
Full table
Full table
Results
In this study, no deaths occurred among the 128 patients.
Findings for the omentum majus embedding group were as follows: grade 3 and above anastomotic stenosis occurred in 12 cases (18.75%), and cervical anastomotic fistula occurred in 6 cases (9.38%). All patients with severe stenosis improved after balloon dilation, and all patients with anastomotic fistula were cured after incision drainage, dressing change, fasting, and anti-infection therapy. Findings for the Neoveil® embedding group were as follows: grade 3 and above anastomotic stenosis occurred in 13 patients (20.31%). No cervical anastomotic fistula occurred in the enrolled patients (0), and all stenosis patients improved after balloon dilatation.
According to the Stooler classification, no significant differences were observed between patients with stenosis and patients with grade 2 or below stenosis (P>0.05) in terms of choking sensation (t=0.21, P=0.647), drinking (t=2.47, P=0.116), hypertension (t=0.47, P=0.493), CHD (t=0.12, P=0.729), diabetes mellitus (t=0.12, P=0.729), postoperative calcium concentration (t=1.17, P=0.262), platelet concentration (t=1.05, P=0.420), BMI (t=1.03, P=0.458), anastomotic fistula (t=0.12, P=0.729), preoperative adjuvant chemotherapy (t=0.89, P=0.345), postoperative adjuvant chemotherapy (t=1.74, P=0.187) or postoperative cough (t=0.15, P=0.696). The differences were significant (P<0.01) regarding scar diathesis (t=18.12, P=0.00002) and reflux (t=17.87, P=0.0002). Reflux (t=7.002, P=0.008) and scar diathesis (t=5.361, P=0.021) in the omentum majus embedding group and reflux (t=5.336, P=0.021) and scar diathesis (t=9.389, P=0.002) in the Neoveil® embedding group were likely the risk factors for anastomotic stenosis.
Comparison of related factors between the Neoveil® group and the omental stenosis group revealed no significant differences (P>0.05) in choking sensation (P1, P2>0.05), hypertension (P1, P2>0.05), diabetes mellitus (P1, P2>0.05), postoperative calcium concentration (P1, P2>0.05), platelet concentration (P1, P2>0.05), BMI (P1, P2>0.05), anastomotic fistula (P1, P2>0.05), preoperative adjuvant chemotherapy (P1, P2>0.05), postoperative adjuvant chemotherapy (P1, P2>0.05) or postoperative cough (P1, P2>0.05). It is possible that these factors had little effect on anastomotic stenosis. However, drinking (P1>0.05, P2<0.05) may be a risk factor for stenosis in omentum majus embedding. The differences were significant for reflux (P1, P2<0.05) and scar diathesis (P1, P2<0.05), which were likely risk factors for stenosis in both groups.
The distribution of the effect of embedding materials on anastomotic fistula and stenosis showed significant differences in patients with anastomotic fistula (χ2=4.372, P<0.05), and the Neoveil® group was superior to the omentum majus group. The difference in embedding materials in patients with anastomotic stenosis was not significant (χ2=0.05, P>0.05).
Discussion
Esophageal cancer is the most common malignancy of the digestive tract. According to a survey of 177 cancer registries in China in 2011, the incidence of esophageal cancer is 0.022%, and the mortality rate is 0.016%. During the same period, the standardized incidence of esophageal cancer worldwide was 0.016%, and the mortality was 0.012% (11). Morbidity and mortality have been on the rise in recent years (12). Currently, surgical resection is still the first choice for the treatment of esophageal cancer. Radical resection of esophageal cancer mainly involves Sweet (13), Ivor-Lewis (14), and Mckeown (15) methods, among which Mckeown has gradually become the predominant technique in recent years (16). With the continuous development and improvement of endoscopic techniques, traditional thoracotomy is gradually being retired from use. Compared with traditional surgical techniques, endoscopic radical resection of esophageal cancer offers vital advantages in reducing postoperative symptoms, shortening hospital stay, and alleviating pain in patients (17).
Anastomotic stenosis and fistula are the most common postoperative complications of esophageal cancer (18). These two complications not only lead to a low quality of life of patients but also endanger their lives (19). Therefore, it is extremely important to reduce the risk factors of anastomotic stenosis and fistula, and it is particularly urgent to find new embedding materials. In this study, Neoveil® was applied in the embedding of cervical anastomosis of esophageal cancer as a new method to reduce postoperative cervical anastomotic stenosis and fistula of esophageal cancer.
Neoveil® is a new type of hemostasis reinforcement material processed with absorbable polyglycolic acid (PGA) as the raw material (20). It is made into sheets or tubes according to different usages and has been widely used in various lung surgeries since its advent. This product is mainly used to reinforce the suture site and prevent air leakage at the lung incision, and it has been widely used in the repair of bronchopleural fistula (21) and various refractory pneumothoraces (22). The effect of tubular Neoveil® combined with an automatic cutting instrument in cutting lung tissue can effectively reduce the incidence of postoperative air leakage at the incision site (23,24). Furthermore, its high biocompatibility can effectively reduce the immune response induced by it being a foreign body (25). Scholars have attempted to apply Neoveil® in the reinforcement and stabilization of the liver, the intestinal tract, glands, meninges, and other vulnerable tissues, the results of which have shown that its performance is better than that of traditional materials, with a significantly reduced postoperative infection rate (26-30). Another study suggested that the rate of anastomotic fistula could be reduced by using Neoveil® as the support material (31).
Cicatricial constitution may be one of the risk factors for anastomotic stenosis in the neck, and is in no way related to the kind of embedding materials used. Scar constitution refers to the possibility of even minor trauma causing the formation of skin tissue larger than the original wound, leading to a keloid scar that does not easily fade (32). Scar constitution is related to certain genetic factors, but there are still no specific indicators to diagnose this condition, and it can only be diagnosed according to the clinical manifestations in patients. The size of the scar is related to the degree of injury, allergic constitution, and blood supply to the injured region. Deep and large wounds will cause strong immune responses, and people with allergies are more likely to experience a strong immune response, resulting in the accumulation of immune cells; additionally, excessive proliferation of fibroblasts is an important mechanism for scar growth (33,34). The venous reflux system at the injured site is closely related to scar formation. In general, poor venous reflux is more likely to cause scar enlargement and growth. The original venous reflux path after esophageal reconstruction is mostly cut off, and continuous hyperemia at the anastomotic site and strong inflammatory reactions may be important causes of stenosis (35).
Gastroesophageal reflux is one of the most common complications after digestive tract reconstruction (36), and it may also be a high-risk factor for cervical anastomotic stenosis according to data. At present, thoracoscopy combined with laparoscopy to free the thoracic esophagus and abdominal proximal gastric body, along with the final external reconstruction with tubular stomach tissue, are relatively mature surgical methods (37). However, after resection of the proximal gastric body, the anti-reflux mechanism is lost, and digestive juices of the patient can easily migrate upward when the patient is lying flat (38). Digestive juices are highly acidic, and the newly formed granular tissue at the esophagogastric anastomosis is more vulnerable to corrosion (39). The damaged site will cause an inflammatory response, which will lead to tissue deposition on the anastomotic site and eventually narrow the anastomotic site.
Data show that different embedding materials have little effect on anastomotic stenosis in the neck, and internal factors of the human body may be an important cause of stenosis. Embedding materials wrapped around the outside of the official cavity have little effect on the stenosis inside the official cavity (4).
Anastomotic fistula is related to the embedding material, and Neoveil® has been shown to be superior to the omentum majus in preventing anastomotic fistula. Pedicled omentum majus is an excellent embedding material, is derived from the patient, and has no possibility of rejection (40,41). However, the omentum majus is prone to fat liquification, and it is difficult for the incision to heal in the liquid, which also increases the risk of anastomotic infection and fistula (42). After the occurrence of fistula, the omentum majus may not be able to form a strict isolating layer to prevent the spread of inflammation. Neoveil® can not only ensure efficacy but also overcome the limitations of traditional embedding materials. It can effectively strengthen the anastomotic site and simultaneously form a barrier against stimulation from the external exudate. Even if microfistula occurs after surgery, it can be wrapped locally, which is conducive to the healing mechanism of fistula (43,44).
Currently, Neoveil® is mainly used in the field of lung surgery. In this study, a limited attempt was made to apply Neoveil® to the embedding of the anastomotic site in the neck of esophageal cancer patients, and its value in clinical application needs to be explored and confirmed by more studies.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This retrospective study was approved by the Medical Ethics Committee of the first Affiliated Hospital of Zhengzhou University, and the requirement for informed patient consent was waived due to the retrospective nature of this study.
References
- Jeon HW, Park JK, Song KY, et al. High Intrathoracic Anastomosis with Thoracoscopy Is Safe and Feasible for Treatment of Esophageal Squamous Cell Carcinoma. PLoS One 2016;11:e0152151. [Crossref] [PubMed]
- Akiyama Y, Iwaya T, Endo F, et al. Stability of cervical esophagogastrostomy via hand-sewn anastomosis after esophagectomy for esophageal cancer. Dis Esophagus 2017;30:1-7. [Crossref] [PubMed]
- van Workum F, Bouwense SAW, Luyer MDP, et al. Intrathoracic versus Cervical ANastomosis after minimally invasive esophagectomy for esophageal cancer: study protocol of the ICAN randomized controlled trial. Trials 2016;17:505-509. [Crossref] [PubMed]
- Wen RZ, Chen MW, Xing L, et al. The investigation of methods for prevention of anastomotic stenosis after resection of esophageal cancer. China Oncol 2016;26:552-5.
- Pierie JP, Graaf PWD, Poen H, et al. Incidence and management of benign anastomotic stricture after cervical oesophagogastrostomy. Br J Surg 1993;80:471-4. [Crossref] [PubMed]
- Hagens ERC, Anderegg MCJ, Van BHMI, et al. International Survey on the Management of Anastomotic Leakage After Esophageal Resection. Ann Thorac Surg 2018;106:1702-8. [Crossref] [PubMed]
- Chen C, Yu Z, Jin Q, et al. Clinical features and risk factors of anastomotic leakage after radical esophagectomy. Zhonghua Wai Ke Za Zhi 2015;53:518-21. [PubMed]
- Allaix ME, Herbella FA, Patti MG. Hybrid trans-thoracic esophagectomy with side-to-side stapled intra-thoracic esophagogastric anastomosis for esophageal cancer. J Gastrointest Surg 2013;17:1972-9. [Crossref] [PubMed]
- Huang J, Zhou Y, Wang C, et al. Logistic regression analysis of the risk factors of anastomotic fistula after radical resection of esophageal-cardiac cancer. Thorac Cancer 2017;8:666-71. [Crossref] [PubMed]
- Kuwata T, Shinohara S, Takenaka M, et al. The impact of covering the bulla with an absorbable polyglycolic acid (PGA) sheet during pneumothorax surgery. Gen Thorac Cardiovasc Surg 2016;64:558-60. [Crossref] [PubMed]
- Zeng H, Zheng R, Zhang S, et al. Esophageal cancer statistics in China, 2011: Estimates based on 177 cancer registries. Thorac Cancer 2016;7:232-7. [Crossref] [PubMed]
- Raymond DP, Seder CW, Wright CD, et al. Predictors of Major Morbidity or Mortality After Resection for Esophageal Cancer: A Society of Thoracic Surgeons General Thoracic Surgery Database Risk Adjustment Model. Ann Thorac Surg 2016;102:207-14. [Crossref] [PubMed]
- Wenxiang W, Baihua Z, Xu L, et al. Minimally invasive esophagectomy via Sweet approach in combination with cervical mediastinoscopy for esophageal squamous cell carcinoma. Int J Surg Oncol 2017;2:45-9. [Crossref]
- Li H, Hu B, You B, et al. Combined laparoscopic and thoracoscopic Ivor Lewis esophagectomy for esophageal cancer: Initial experience from China. Chin Med J 2012;125:1376-80. [PubMed]
- Mu JW, Gao SG, Xue Q, et al. Comparison of short-term outcomes and three year survival between total minimally invasive McKeown and dual-incision esophagectomy. Thorac Cancer 2017;8:80-7. [Crossref] [PubMed]
- Sun HB, Li Y, Liu XB, et al. Early Oral Feeding Following McKeown Minimally Invasive Esophagectomy: An Open-label, Randomized, Controlled, Noninferiority Trial. Ann Surg 2018;267:435-42. [Crossref] [PubMed]
- Moon DH, Lee JM, Jeon JH, et al. Clinical outcomes of video-assisted thoracoscopic surgery esophagectomy for esophageal cancer: a propensity score-matched analysis. J Thorac Dis 2017;9:3005-12. [Crossref] [PubMed]
- Guyton KL, Hyman NH, Alverdy JC. Prevention of Perioperative Anastomotic Healing Complications: Anastomotic Stricture and Anastomotic Leak. Adv Surg 2016;50:129-41. [Crossref] [PubMed]
- Yamana I, Takeno S, Yamada T, et al. The Risk Factors for Refractory Fistula after Esophagectomy with Gastric Tube Reconstruction in Patients with Esophageal Cancer. Dig Surg 2017;34:18-24. [Crossref] [PubMed]
- Nagami Y, Shiba M, Arakawa T. Use of PGA Sheets in the Endoscopic Closure of a Perforation After Endoscopic Submucosal Dissection for Gastric-Tube Cancer. Am J Gastroenterol 2016;111:768. [Crossref] [PubMed]
- Xu K, Ye B. The application of NEOVEIL in bronchopleural fistula repair. Zhe Jiang J Clin Med 2006;8:692.
- Luo ZQ, Huang J. The application of neovil in surgical treatment of patients with COPD complicated with spontaneous pneumothorax. Contemp Med 2014;27:51-2.
- Wang JJ, Chen SH, Li GM, et al. The effect of thoracoscopic surgery combined with tubular neovil gasket in the treatment of spontaneous pneumothorax. Shanxi Med J 2016;45:834-6.
- Takamochi K, Oh S, Miyasaka Y, et al. Prospective randomized trial comparing buttressed versus nonbuttressed stapling in patients undergoing pulmonary lobectomy. Thorac Cardiovasc Surg 2014;62:696-704. [Crossref] [PubMed]
- Takimoto K, Imai Y, Matsuyama K. Endoscopic tissue shielding method with polyglycolic acid sheets and fibrin glue to prevent delayed perforation after duodenal endoscopic submucosal dissection. Dig Endosc 2014;26:46-9. [Crossref] [PubMed]
- Han S, Chung H, Park JC, et al. Endoscopic Management of Gastrointestinal Leaks and Perforation with Polyglycolic Acid Sheets. Clin Endosc 2017;50:293-6. [Crossref] [PubMed]
- Kimura M, Kuwabara Y, Taniwaki S, et al. Improving the side-to-side stapled anastomosis: comparison of staplers for robust crotch formation. Surg Obes Relat Dis 2018;14:16-21. [Crossref] [PubMed]
- Naito M, Sato T, Nakamura T, et al. Secure overlap stapling using a linear stapler with bioabsorbable polyglycolic acid felt. Asian J Endosc Surg 2017;10:308-12. [Crossref] [PubMed]
- Aizawa T, Kuwabara M, Kubo S, et al. Polyglycolic Acid Felt for Prevention of Frey Syndrome After Parotidectomy. Ann Plast Surg 2018;81:438-40. [Crossref] [PubMed]
- Terasaka S, Taoka T, Kuroda S, et al. Efficacy and safety of non-suture dural closure using a novel dural substitute consisting of polyglycolic acid felt and fibrin glue to prevent cerebrospinal fluid leakage—A non-controlled, open-label, multicenter clinical trial. J Mater Sci Mater Med 2017;28:69. [Crossref] [PubMed]
- Kimura M, Terashita Y. Use of bioabsorbable staple reinforcement material in side-to-side anastomoses: Suture line reinforcement of the weak point of the anastomosis. Ann Med Surg (Lond) 2016;6:50-5. [Crossref] [PubMed]
- Burd A, Huang L. Hypertrophic response and keloid diathesis: two very different forms of scar. Plast Reconstr Surg 2005;116:150e-7e. [Crossref] [PubMed]
- Hu X, Wang HT, Liu JQ, et al. The role of ERK and JNK signaling in connective tissue growth factor induced extracellular matrix protein production and scar formation. Arch Dermatol Res 2013;305:433-45. [Crossref] [PubMed]
- Hsieh SC, Wu CC, Hsu SL, Yen JH. Molecular mechanisms of gallic acid-induced growth inhibition, apoptosis, and necrosis in hypertrophic scar fibroblasts. Life Sci 2017;179:130-8. [Crossref] [PubMed]
- Li M, Zhu C, Zhao G, et al. Application of laparoscopic function-preservation proximal gastrectomy in the treatment of early gastric cancer. Zhonghua wei chang wai ke za zhi 2016;19:190-4. [PubMed]
- Ying KM, Chen Z, Dang CX, et al. Clinicopathology and Survival in Patients with Gastroesophageal Reflux After Radical Surgery of Proximal Gastric Cancer. Dig Dis Sci 2018;63:1035-42. [Crossref] [PubMed]
- Zhang R, Wang P, Zhang X, et al. Gastric tube reconstruction prevents postoperative recurrence and metastasis of esophageal cancer. Oncol Lett 2016;11:2507-9. [Crossref] [PubMed]
- Oh HJ, Choi MG, Park JM, et al. Acid Secretion and Its Relationship to Esophageal Reflux Symptom in Patients with Subtotal Gastrectomy. Dig Dis Sci 2018;63:703-12. [Crossref] [PubMed]
- Tabola R, Augoff K, Lewandowski A, et al. Esophageal anastomosis - how the granulation phase of wound healing improves the incidence of anastomotic leakage. Oncol Lett 2016;12:2038-44. [Crossref] [PubMed]
- Ishibashi Y, Fukunaga T, Mikami S, et al. Triple-stapled quadrilateral anastomosis: a new technique for creation of an esophagogastric anastomosis. Esophagus 2018;15:88-94. [Crossref] [PubMed]
- Bruzoni M, Steinberg GK, Dutta S. Laparoscopic harvesting of omental pedicle flap for cerebral revascularization in children with moyamoya disease. J Pediatr Surg 2016;51:592-7. [Crossref] [PubMed]
- Cai Y, Zhou Y, Li Z, et al. Surgical outcome of laparoscopic colectomy for colorectal cancer in obese patients: A comparative study with open colectomy. Oncol Lett 2013;6:1057-62. [Crossref] [PubMed]
- Naito M, Yamanashi T, Nakamura T, et al. Safety and efficacy of a novel linear staple device with bioabsorbable polyglicolic acid felt in laparoscopic colorectal surgery. Asian J Endosc Surg 2017;10:35-9. [Crossref] [PubMed]
- Naito M, Miura H, Nakamura T, et al. Sutureless functional end-to-end anastomosis using a linear stapler with polyglycolic acid felt for intestinal anastomoses. Ann Med Surg (Lond) 2017;17:50-3. [Crossref] [PubMed]