
S-1 salvage chemotherapy for stage IV thymic carcinoma: a study of 44 cases
Introduction
Thymic carcinoma (TC) is a rare mediastinal tumor and accounts for about 14% of all thymic epithelial tumors (1). Compared with thymoma, TC is more likely to invade neighboring tissues and metastasize to distant organs. TC was, therefore, classified as a distinct entity from thymoma in the 2004 World Health Organization (WHO) classification (2).
Systemic chemotherapy remains the mainstay of treatment for advanced TC, although no standard chemotherapeutic regimen has been established due to its rarity. Several reports have shown the efficacy of cisplatin-based chemotherapy such as ADOC (cisplatin, doxorubicin, vincristine, and cyclophosphamide) and CODE (cisplatin, vincristine, doxorubicin, and etoposide) (3-4). In recent years, the combination of taxane (paclitaxel, docetaxel, or genexol-PM) with platinum has also shown efficacy in the management of TC (5-7). Based on the above results, these regimens are recommended as front-line chemotherapy for advanced TCs. However, what constitutes optimal treatment when first-line chemotherapy fails, or the patient’s performance status is too poor to tolerate high-density chemotherapy remains controversial.
S-1 is an orally active combination of tegafur (a prodrug of 5-fluorouracil) with gimeracil and oteracil. With the addition of the two modulators, the anti-tumor activity of tegafur is increased while the gastrointestinal toxicity is reduced. As of now, there are only a few case reports (8-11) investigating the efficacy of S-1 monotherapy for relapsed TC, and none included more than 5 patients. In this study, we evaluated the efficacy and safety of S-1 in 44 patients with relapsed TC, in order to better understand how to allocate candidates who would benefit from this oral agent properly.
Methods
Patient selection
In this retrospective study, the patients presented were all treated with S-1 from January 2013 to July 2017 in our institution. All patients satisfied the following eligibility criteria: (I) histologically confirmed TC; (II) measurable lesions on image examinations; (III) previously treated by front-line chemotherapy; (IV) age of less than 80 years; (V) Eastern Cooperative Oncology Group Performance Scale status (ECOG PS) of 0-2; (VI) adequate bone marrow, hepatic, and renal function.
Treatment methods
The S-1 capsules (20 mg/capsule, produced by Lunan Pharmacy Co., Ltd., Shandong, China) were taken orally twice a day for 2 weeks, followed by a 1-week drug-free interval. There were three different dose levels (80, 100, and 120 mg), which were basically prescribed according to body surface area (BSA): 80 mg for BSA <1.25 m2, 100 mg for 1.25< BSA <1.5 m2, and 120 mg for BSA >1.5 m2. The dose, however, could be reduced to a lower level when the patient’s PS was judged as 2. Patients were advised to take capsules after meals for less gastrointestinal side effects. The cycles would be repeated unless disease progressed or intolerable adverse effect occurred. This study was approved by the Shanghai Chest Hospital Ethics Committee (ID No. KS1920). All patients gave their informed consent before taking part in this study.
Evaluation of response and toxicity
Computed tomography (CT) scan was performed every two cycles to evaluate the response status. All patients in this study were in the late stage, and the diseases often spread extensively; therefore, we adopted a new Response Evaluation Criteria in Solid Tumors (RECIST) guideline for the evaluation, which was proposed by the International Thymic Malignancy Interest Group (ITMIG) (12). The new guideline provides effective methods to calculate the response rate of complicated lesions, like multiple pleural implants or pulmonary metastasis. The adverse events were recorded and graded using the Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0).
Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method. The PFS was calculated from the initiation of S-1 until the date of disease progression or death from any cause. The OS was defined as the interval between the starting of S-1 and the date of death or the last follow-up. All statistical analyses were performed with SPSS 16.0. All tests were two-sided, and P values less than 0.05 were considered statistically significant.
Results
Patient characteristics
Between January 2013 and July 2017, a total of 44 patients were included in this study with a balanced gender distribution (22:22). The clinical profiles of the 44 patients are summarized in Table 1. Squamous cell carcinoma was the dominant histology type. Most patients were in stage IVb. The common failure sites were the lung (n=21), pleura (n=15), lymph node (n=7), liver (n=4), bone (n=3), brain (n=2), and pericardium (n=1). Seven patients had multiple organ metastases. All patients received first-line chemotherapy. Second-line chemotherapy was also applied in 22 (50%) patients. There were even 5 (11%) patients who received third-line chemotherapy before S-1. The regimens adopted in front-line chemotherapy are pooled in Table 2.

Full table
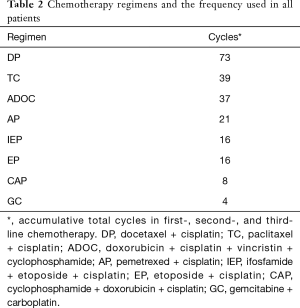
Full table
Response and survival
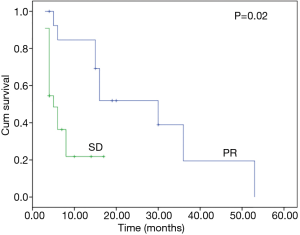
S-1 was prescribed at a dose of 80 mg for 18 patients (41%), 100 mg for 17 patients (38%), and 120 mg for 9 patients (21%). The median number of cycles of administrated per patient was 3 [1–32]. Out of the total of 44 patients, 13 (30%) achieved a partial response after the first evaluation. Tumors of 22 (50%) patients remained stable during the cycles. By contrast, tumors of the other 9 (20%) patients showed a rapid progression. The response rates in different sites are summarized in Table 3. The median PFS and OS for the whole group were 6 (95% CI, 7.0–13.9) months and 15 (95% CI, 13.2–21.6) months, respectively (Figures 1,2). For the 13 patients who showed response to S-1, the median PFS was 22 (95% CI, 15.5–30) months (Figure 3).

Full table
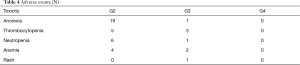
Toxicity
The toxicities in 44 patients are summarized in Table 4. No treatment-related deaths occurred. Appetite loss was the most common side effect, but most of them were mild and tolerable. Treatment was stopped in one patient after 4 cycles of S-1 due to a grade 3 neutropenia, which could not be improved by injection of granulocyte. Three patients had their drug intake suspended for 2–3 weeks due to grade 3 thrombocytopenia, and then resumed taking S-1 when their blood count returned to normal. One patient developed a grade 3 skin rash, which regressed gradually after a drug suspension.
Full table
Discussion
In the current study, S-1 monotherapy demonstrated an ORR of 29% and a median PFS of 5 months with low toxicity in 44 patients, which suggested that S-1 could be applied as an effective salvage therapy for late-staged TC.
Due to the rarity of thymic neoplasm, the chances for randomized prospective trials are dismal. Thus, the optimal chemotherapeutic regimen for TC remains controversial. Several reports have shown the efficacy of cisplatin and anthracycline-based regimen, like ADOC and CODE in TC, with an ORR ranging from 42–75%. In recent years, new regimens have also been tested for TC. For instance, carboplatin plus paclitaxol, which has been proven successful in non-small cell lung cancer (NSCLC) yielded a promising ORR of 36% with lower toxicity. All the above regimens are currently recommended as front-line chemotherapies for TC by a few guidelines. However, these anti-cancer therapies cannot be endlessly repeated when patients’ tolerability and dose-related cardiac toxicity of anthracycline are considered. Unfortunately, there are few reports so far investigating the second-line or beyond chemotherapy when previous regimens fail. Generally, patients’ performance status deteriorates significantly after a series of intensive chemotherapy sessions, which makes an effective salvage therapy with low toxicity warranted when the disease progresses again.
S-1, a novel oral fluoropyrimidine agent, was invented in the early 1990s. The anticancer mechanism of S-1 has been clearly elaborated in previous reports (13). In a series of clinical trials, S-1 yielded an ORR of 40–60% in gastric cancer (14,15) and 36% in advanced colorectal cancer (16). From 1997, S-1 has gradually found approval for the treatment of gastric cancer, head and neck cancer, colorectal cancer, NSCLC, and other diseases. In 2008, Ono et al. (10) was first to report the efficacy of S-1 on TC in the English language literature. After this, several authors also explored S-1 monotherapy in TC, but none of the studies included more than 4 patients. Thus, it is difficult to arrive at a convincing conclusion about the efficacy and toxicity of this treatment. In the present study, 44 patients were enrolled, which is a greater sample than the sum of all the previous reports. The ORR of the whole group was 30% (13/44), which is no worse than other single agents (31% for docetaxel, 11% for pemetrexed, 44% for amrubicin, 12.5% for etoposide and 0% for ifosfamide) (17-21). The median PFS at 6 months was also similar. It should be especially noted that the toxicity of S-1 was milder and more tolerable, which is consistent with the result of S-1 on other solid tumors. The high acceptability of this agent enables several responding patients to resume S-1 for more than even 2 years.
Thus far, there has been no specific indicator identified (biological or clinical) that can help predict the sensitivity of TC to S-1. In some studies (22,23), thymidylate synthase (TS) is regarded as a predictive biomarker, and the lower levels of TS protein, mRNA, or activity are associated with greater efficacy for S-1. However, its role in TC needs to be clarified further.
Our study indicated that lung metastasis and pleural nodules were more sensitive to S-1 therapy, with an ORR of 38% and 40%, respectively (Table 3). When we examined the previous studies investigating S-1 on TC (8-11), we also found that, in the responding patients, all histology types were squamous cell carcinoma and most of the failure sites were lung metastasis. These findings imply that patients with pulmonary and pleural metastasis might benefit more from S-1 salvage therapy. As is shown in Figure 3, patients who showed response to S-1 had a PFS as long as 22 months, so it is very critical to select potential beneficiaries using predictive biomarkers like TS, or other image clues.
In recent years, target therapy and immunotherapy have become popular research areas in anti-cancer treatment, and some authors have extended this investigation into TC. In Thomas’ report (24), sunitinib produced a 26% response rate in TC, while in Giaccone’s study (25), the response rate after pembrolizumab treatment was 22.5%. Compared with the above results, the efficacy of S-1 appears satisfactory.
In conclusion, S-1 is an effective monotherapy for advanced TC and shows low toxicity. Further prospective multi-institutional studies for this treatment are warranted.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Shanghai Chest Hospital Ethics Committee (ID No. KS1920). All patients gave their informed consent before taking part in this study.
References
- Kondo K, Monden Y. Therapy for thymic epithelial tumors: a clinical study of 1,320 patients from Japan. Ann Thorac Surg 2003;76:878-84. [Crossref] [PubMed]
- Ströbel P, Marx A, Zettl A, et al. Thymoma and thymic carcinoma: an update of the WHO Classification 2004. Surg Today 2005;35:805-11. [Crossref] [PubMed]
- Koizumi T, Takabayashi Y, Yamagishi S, et al. Chemotherapy for advanced thymic carcinoma: clinical response to cisplatin, doxorubicin, vincristine, and cyclophosphamide (ADOC chemotherapy). Am J Clin Oncol 2002;25:266-8. [Crossref] [PubMed]
- Yoh K, Goto K, Ishii G, et al. Weekly chemotherapy with cisplatin, vincristine, doxorubicine, and etoposide is an effective treatment for advanced thymic carcinoma. Cancer 2003;98:926-31. [Crossref] [PubMed]
- Hirai F, Yamanaka T, Taguchi K, et al. West Japan Oncology Group. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015;26:363-8. [Crossref] [PubMed]
- Park S, Ahn MJ, Ahn JS, et al. A prospective phase II trial of induction chemotherapy with docetaxel/cisplatin for Masaoka stage III/IV thymic epithelial tumors. J Thorac Oncol 2013;8:959-66. [Crossref] [PubMed]
- Kim HS, Lee JY, Lim SH, et al. A prospective phase II study of cisplatin and cremophor EL-free paclitaxel (genexol-PM) in patients with unresectable thymic epithelial tumors. J Thorac Oncol 2015;10:1800-6. [Crossref] [PubMed]
- Koizumi T, Agatsuma T, Komatsu Y, et al. Successful S-1 monotherapy for chemorefractory thymic carcinoma. Anticancer Res 2011;31:299-301. [PubMed]
- Okuma Y, Shimokawa T, Takagi Y, et al. S-1 is an active anticancer agent for advanced thymic carcinoma. Lung Cancer 2010;70:357-63. [Crossref] [PubMed]
- Ono A, Naito T, Yamamoto N. S-1 treatment for chemorefractory thymic carcinoma. J Thorac Oncol 2008;3:1076. [Crossref] [PubMed]
- Hirai F, Seto T, Inamasu E, et al. Results of S-1-based chemotherapy for platinum (and anthracycline)-refractory advanced thymic carcinoma. Anticancer Res 2014;34:5743-7. [PubMed]
- Benveniste MF, Korst RJ, Rajan A, et al. A practical guide from the International Thymic Malignancy Interest Group (ITMIG) regarding the radiographic assessment of treatment response of thymic epithelial tumors using modified RECIST criteria. J Thorac Oncol 2014;9:S119-24. [Crossref] [PubMed]
- Shirasaka T. Development history and concept of an oral anticancer agent s-1 (ts-1): its clinical usefulness and future vistas. Jpn J Clin Oncol 2009;39:2-15. [Crossref] [PubMed]
- Horikoshi N, Mitachl Y, Sakata Y, et al. S-1, new oral fluoropyrimidine, is very active in patients with advanced gastric cancer (early phase II study). Proc Am Soc Clin Oncol 1996;15:206.
- Sakata Y, Ohtsu A, Horikoshi N, et al. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer 1998;34:1715-20. [Crossref] [PubMed]
- Baba H, Ohtsu A, Sakata Y, et al. Late phase II study of S-1 in patients with advanced colorectal cancer in Japan. Proc Am Soc Clin Oncol 1998;17:277a.
- Watanabe N, Umemura S, Niho S, et al. Docetaxel for platinum-refractory advanced thymic carcinoma. Jpn J Clin Oncol 2015;45:665-9. [Crossref] [PubMed]
- Liang Y, Padda SK, Riess JW, et al. Pemetrexed in patients with thymic malignancies previously treated with chemotherapy. Lung Cancer 2015;87:34-8. [Crossref] [PubMed]
- Hirai F, Seto T, Yamanaka T, et al. Amrubicin as second-line and beyond treatment for platinum-refractoryadvanced thymic carcinoma. Jpn J Clin Oncol 2013;43:1018-22. [Crossref] [PubMed]
- Celine B, Florence F, Benjamin B. Oral etoposide in pretreated advanced thymoma and thymic carcinoma. a French experience. J Thorac Oncol 2013;8:39-40.
- Highley MS, Underhill CR, Parnis FX, et al. Treatment of invasive thymoma with single-agent ifosfamide. J Clin Oncol 1999;17:2737-44. [Crossref] [PubMed]
- Yasumatsu R, Nakashima T, Uryu H, et al. Correlations between thymidylate synthase expression and chemosensitivity to 5-fluorouracil, cell proliferation and clinical outcome in head and neck squamous cell carcinoma. Chemotherapy 2009;55:36-41. [Crossref] [PubMed]
- Obara S, Yamamoto K, Hosogai N, et al. Evaluation of TS-1 based treatment and expression of thymidylate synthase and dihydropyrimidine dehydrogenase on oral squamous cell carcinoma. Oral Oncol 2005;41:276-82. [Crossref] [PubMed]
- Thomas A, Rajan A, Berman A, et al. Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015;16:177-86. [Crossref] [PubMed]
- Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018;19:347-55. [Crossref] [PubMed]




