The lung adenocarcinoma guidelines: what to be considered by surgeons
Introduction
Lung adenocarcinoma is the most common type of primary lung cancer, accounting for almost half of these tumors. Although advances have taken place in oncology, molecular biology, pathology, radiology, and surgery of lung adenocarcinoma during the past few decades, the histologic subclassification of this type of cancer has remained difficult because of the its heterogenous nature. In this context, in 2011 the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) (1,2), have proposed a new subclassification of lung adenocarcinomas.
The 1999 World Health Organization (WHO) classification of lung tumors introduced the adenocarcinoma of mixed subtype category, partly due to a change in definition of bronchioloalveolar carcinoma (BAC) (3,4) and this subtype was retained in the 2004 WHO. Since the publication of 1999/2004 WHO classifications, there have been many advances in the practice and understanding of lung adenocarcinoma that should be included in a new classification.
In the new classification, BAC is called adenocarcinoma in situ (AIS) and describes small (<3 cm) solitary lesions with 100% lepidic growth. A related entity, previously sometimes referred to as minimally invasive BAC was not included in 1999/2004 WHO classifications but is introduced in the new classification and called minimally invasive adenocarcinoma (MIA). MIA describes small (<3 cm) solitary adenocarcinomas with predominant lepidic growth and <5 mm invasion. If resected, both AIS and MIA are associated with 100% or near 100% disease-free survival. This type of cancer are usually nonmucinous, although rare examples of mucinous AIS and mucinous MIA can be detected (3,5,6). However, as the new classification enters common use, the committee has offered the suggestion that BAC may be retained in temporary use by referring to it as “formerly BAC” in any setting for which this clarification might be helpful.
Also, this new classification was founded on an evidence-based approach to a systematic review of 11,368 citations from the related literature (3). Validation has involved projects relating to histologic and cytologic analysis of small biopsy specimens (7), histologic subtyping, grading, and observer variation among expert pathologists (8).
Pathologists now play an important role in personalized medicine for patients with lung cancer as a result of the newly recognized importance of histologic classification and molecular testing in stratifying patients for specific therapies. This classification provides a standard for pathologic diagnosis not only for patient care but also for clinical trials.
The project
This new classification has been made as a collaborative effort of the IASLC, the ATS, and the ERS. The purpose is to provide an integrated clinical, radiological, molecular, and pathological approach to classification of the various types of lung adenocarcinoma. A systematic review was performed by guidance by members of the ATS Documents Development and Implementation Committee. The search were performed in June 2008 with an update in June 2009 resulting in 11,368 citations, which were reviewed to exclude articles that did not have any importance for lung adenocarcinoma classification. The articles (total =312) were reviewed for specific data, and based on analysis of tables from this systematic approach, recommendations were made according to the Grades of Recommendation, Assessment, Development, and Evaluation (GRADE) (3). The GRADE system has two major components, including grading the strength of the recommendation, and evaluating the quality of the evidence. After review of all articles, the committee developed recommendations after a multidisciplinary discussion.
Regarding lung adenocarcinoma heterogeneity, confusion is unavoidable in practice. Clinical studies are difficult to compare and a need for universally accepted criteria for adenocarcinoma subtypes still remains, in particular for tumors formerly classified as BAC. As enormous resources are being spent on trials involving molecular and therapeutic aspects of adenocarcinoma of the lung, the development of standardized criteria is of great importance and should help advance the field, increasing the impact of research, and improving patient care. This classification is needed to assist in determining patient therapy and predicting outcome.
Histopathology
Primary aims of the new classification include provision of consistent terms and diagnostic criteria for adenocarcinoma subtypes, particularly for BAC and mixed subtype adenocarcinoma, besides and incorporation of significant practical changes in all clinical and diagnostic fields into a classification that is still principally based on histopathologic examination.
There are several major differences in this classification compared with those previously published by the WHO. First, there was a multidisciplinary effort with clinicians, radiologists, molecular biologists and surgeons, rather than a primary effort by pathologists. This led to an emphasis on correlations between pathological, clinical, radiologic and molecular characteristics of tumors that allowed multiple paradigm shifts in the diagnosis and management of patients with lung cancer. Second, it was recognized that 70% of patients with lung cancer present with advanced-stage disease, which is usually diagnosed on the basis of small biopsies and cytology. Because prior WHO classifications focused on lung cancer diagnosis in resection specimens, which are obtained in only 30% of patients, an additional effort was made to define terminology and criteria to be used in small biopsies and cytology. Based on the previous observations, this classification is divided in: (I) small biopsy and cytology specimens for patients with advanced-stage lung cancer (Table 1); (II) resection specimens for early-stage patients who are eligible for surgical resection (Table 2).
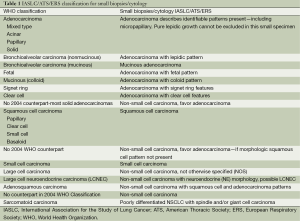
Full table
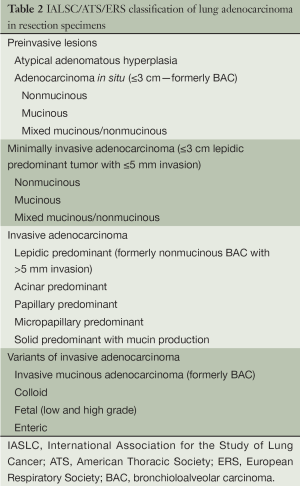
Full table
A major point in this classification is the concept that personalized medicine in advanced lung cancer is determined by histology, and genetics for strategic tissue management of small biopsies is critical not only for diagnosis, but also for molecular studies.
In fact, for patients with advanced non-small cell lung cancer, recent progress in molecular biology and oncology has led to discovery of epidermal growth factor receptor (EGFR) mutation and its prediction of response to EGFR tyrosine kinase inhibitors (TKIs) in adenocarcinoma patients and the requirement to exclude a diagnosis of squamous cell carcinoma to determine eligibility of patients for treatment with pemetrexed, or bevacizumab (9-12).
The emergence of radiologic-pathologic correlations between ground-glass versus solid or mixed opacities seen by computed tomography (CT) and BAC versus invasive growth by pathology, has opened new opportunities for imaging studies and for predicting the histologic subtype of adenocarcinomas and, as a result, preoperative assessment for choice of timing and type of surgical intervention (13-19).
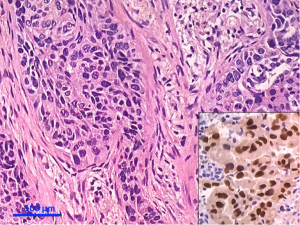
Separate projects were initiated for this classification effort in an attempt to achieve data to test the proposed system. These included projects on small biopsies, histological grading (20,21), stage I adenocarcinomas, small adenocarcinomas from Japan, international multiple pathologists project on reproducibility of recognizing major histologic patterns of lung adenocarcinoma, molecular-histologic correlations (22-24), and radiologic-pathologic correlations focused on AIS and MIA. Those tumors are illustrated on Figures 1-5.
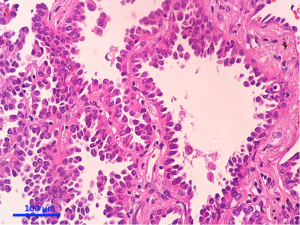
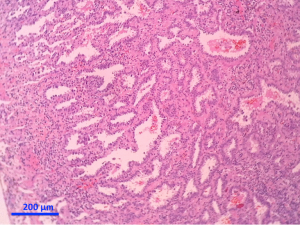
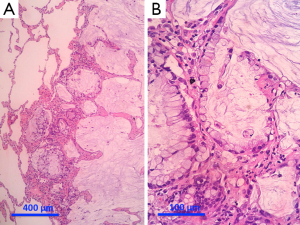
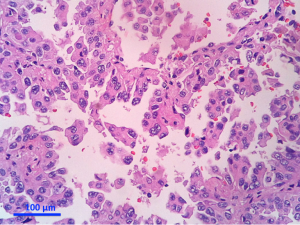
The goal is not only longer provide the most accurate diagnosis but also to manage the tissue in a way that immunohistochemical and/or molecular studies can be performed to obtain predictive and prognostic data that will lead to improvement in patient outcomes. Because of the lack of universal diagnostic criteria in the literature, there is a need for future validation studies based on these standardized pathologic criteria with clinical, molecular, radiologic, and surgical corrections.
Main recommendations were proposed by the committee (3), as follows:
- Discontinue the use of the term BAC;
- Introduction of AIS and MIA, for resection specimens;
- Predominant pattern replaces former mixed subtype in invasive adenocarcinoma;
- Changes in variants of adenocarcinoma;
- Classification guidance for different types of samples (e.g., small biopsies or surgical specimens);
- In case of multiple adenocarcinomas, a histologic subtype may facilitate the comparision between metastases or multiple primary tumors;
- In early-stage adenocarcinoma, the recommendation of micropapillary predominant adenocarcinoma—as a guidance to prognosis.
Discussion
Histopathologic classification systems have traditionally been the domain of pathologists. However, there is a trend for development of classifications systems by multidisciplinary panels of experts. In addition, some entities in this latter classification are now defined by the combination of a morphologic phenotype tightly correlated to a molecular profile. This enables oncologists to recommend individualized therapy, whenever available, to such patients. The shifting paradigm toward individualized therapy has been evolving in lung cancer management over the past decade. An updated, multidisciplinary approach to the classification of lung adenocarcinoma will encourage and enable continued research into the correlation of morphologic phenotype with the underlying molecular profile.
With the advent of helical CT and the screening trials in high-risk populations, there is a renewed interest in small nodules, especially those with ground-glass opacity (GGO). A subject of intensive investigation is the surgical approach of some of these lesions and if they could be treated by limited resection, so-called sublobar resection comprising anatomical segmentectomy and wedge excision. For a limited resection to be oncologically valid, a precise pre- and intra-operative diagnosis becomes imperative. Indeed, specific criteria to AIS and MIA on chest CT, such as percentage GGO, tumor shadow disappearance rate and histogram analysis, have been shown to have a high predictive value (25).
Additionally, the necessity of lobectomy for stage I lung cancer, especially for those tumors of ≤2 cm (cT1a disease), is also being questioned (26). Recently, there have been numerous publications suggesting that sublobar resection for early stage lung cancers may be an adequate surgical treatment. On the other hand, these studies were not randomized and many were retrospective. Most reports showed no difference in survival or in locoregional recurrence between lobectomy and sublobar resection for tumors ≤2 cm. Tumors with a GGO appearance on CT are reported to have 100% survival at 5 years after resection (27,28). However, possible delayed cut-end recurrences have been described after limited resection of GGO lesions (29).
There are still few key points to be considered for the accurate differentiation of AIS and MIA from invasive adenocarcinoma. First, the prognostic significance of possibly aggressive micropapillary or solid components as invasive areas in tumors with MIA histology remains to be determined. There has been reported a minimally invasive micropapillary adenocarcinoma that showed an extensive lymphovascular invasion and lymph node involvement, and the patient died within 16 months with widespread metastases (30). The presence of high grade histology, such as solid or micropapillary components, could perhaps affect the prognosis, even in those patients with MIA. Second, the new IASLC/ATS/ERS classification recommended that for a tumor larger than 3 cm, particularly if it has not been completely sampled, the term lepidic predominant adenocarcinoma is best applied with a comment that the clinical behavior is uncertain and/or that an invasive component cannot be excluded (3). There is insufficient evidence to support the notion that 100% disease-free survival can occur in such tumors with MIA histology >3 cm.
Third, lepidic growth may be composed of neoplastic cells with nuclear atypia resembling that of the adjacent invasive tumor. Some observers would further argue that such lepidic patterns correspond to an aerogenous spread of tumor cells, but are no longer an “in situ” component (31). Because it has not been established that this kind of lepidic growth is not in fact an aerogenous spread of invasive carcinoma, the diagnosis of AIS or MIA should not be made unless the lesion has a discrete circumscribed border; cases with miliary spread of small foci of tumors into adjacent lung parenchyma and/or with lobar consolidation should be excluded (3). This observation is really troubling, because lepidic percent cutoff is associated with good survival.
Another issue regarding surgical approach that is still under investigation is a standard treatment algorithm for multiple lesions. Several factors have to be taken into consideration: number and size of the different nodules, ipsilateral versus contralateral location, primary versus metastatic lesions, and specific nature (atypical adenomatous hyperplasia, AIS or MIA). Conservative treatment with frequent follow-up is advocated for potentially benign lesions. When it is technically not possible to remove multiple, synchronous, pure GGO lesions, regular follow-up with chest CT represents an alternative approach to surgical resection (32). For malignant nodules several options exist, such as lobectomy for same-lobe nodules (now considered to be T3 disease), bilobectomy, lobectomy with wide wedge resection(s), multiple wide wedge resections or segmentectomies, and pneumonectomy, depending on functional capacity. Such resections can sometimes be performed by VATS. One approach is to perform an anatomical resection (segmentectomy or lobectomy) for larger, more invasive or more central tumors, and removing the smaller, peripheral or less invasive tumours by wedge resection. However, such an approach has not yet been validated in clinical studies. For precise diagnosis of metachronous versus synchronous lung cancers, the classical criteria of Martini and Melamed are often used, at the present time combined with molecular genetic analysis (33). To be considered multiple primary tumors, the interval between the two should be >2 years. When the interval is <2 years and both tumors are detected in the same lobe, different histology should be present or they should arise from foci of carcinoma in situ. When the interval is <2 years and they are found in different lobes, there should be no carcinoma cells common to both and no systemic metastases.
Conclusions
The 2011 IASLC/ATS/ERS adenocarcinoma classification can have an impact on TNM staging. It may help in comparing histologic characteristics of multiple lung adenocarcinomas to determine whether they are intrapulmonary metastases versus separate primaries. Use of comprehensive histologic subtyping along with other histologic characteristics has been shown to have good correlation with molecular analyses and clinical behavior (34). Also, it may be more meaningful clinically to measure tumor size in lung adenocarcinomas that have a lepidic component by using invasive size rather than total size to determine the size T factor. Existing data already suggest that this can be applied to CT as well as pathologic assessment of these tumors (35). In the next edition of the TNM, AIS may be regarded as Tis and MIA may be regarded as Tmi.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Devesa SS, Bray F, Vizcaino AP, et al. International lung cancer trends by histologic type: male:female differences diminishing and adenocarcinoma rates rising. Int J Cancer 2005;117:294-9. [PubMed]
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin 2010;60:277-300. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 2011;6:244-85. [PubMed]
- Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer 1995;75:2844-52. [PubMed]
- Yim J, Zhu LC, Chiriboga L, et al. Histologic features are important prognostic indicators in early stages lung adenocarcinomas. Mod Pathol 2007;20:233-41. [PubMed]
- Borczuk AC, Qian F, Kazeros A, et al. Invasive size is an independent predictor of survival in pulmonary adenocarcinoma. Am J Surg Pathol 2009;33:462-9. [PubMed]
- Nicholson AG, Gonzalez D, Shah P, et al. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol 2010;5:436-41. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947-57. [PubMed]
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010;11:121-8. [PubMed]
- Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380-8. [PubMed]
- Zhou C, Wu YL, Chen G, et al. Efficacy results from the randomised phase III OPTIMAL (CTONG 0802) study comparing first-line erlotinib versus carboplatin (CBDCA) plus gemcitabine (GEM), in Chinese advanced non-small-cell lung cancer (NSCLC) patients (pts) with EGFR activating mutations. Ann Oncol 2010;21: abstr LBA13.
- Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer 2001;33:17-25. [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [PubMed]
- Takamochi K, Nagai K, Yoshida J, et al. Pathologic N0 status in pulmonary adenocarcinoma is predictable by combining serum carcinoembryonic antigen level and computed tomographic findings. J Thorac Cardiovasc Surg 2001;122:325-30. [PubMed]
- Sakurai H, Maeshima A, Watanabe S, et al. Grade of stromal invasion in small adenocarcinoma of the lung: histopathological minimal invasion and prognosis. Am J Surg Pathol 2004;28:198-206. [PubMed]
- Adler B, Padley S, Miller RR, et al. High-resolution CT of bronchioloalveolar carcinoma. AJR Am J Roentgenol 1992;159:275-7. [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [PubMed]
- Nakamura H, Kawasaki N, Taguchi M, et al. Survival following lobectomy vs limited resection for stage I lung cancer: a meta-analysis. Br J Cancer 2005;92:1033-7. [PubMed]
- Loo PS, Thomas SC, Nicolson MC, et al. Subtyping of undifferentiated non-small cell carcinomas in bronchial biopsy specimens. J Thorac Oncol 2010;5:442-7. [PubMed]
- Nicholson AG, Gonzalez D, Shah P, et al. Refining the diagnosis and EGFR status of non-small cell lung carcinoma in biopsy and cytologic material, using a panel of mucin staining, TTF-1, cytokeratin 5/6, and P63, and EGFR mutation analysis. J Thorac Oncol 2010;5:436-41. [PubMed]
- Barletta JA, Perner S, Iafrate AJ, et al. Clinical significance of TTF-1 protein expression and TTF-1 gene amplification in lung adenocarcinoma. J Cell Mol Med 2009;13:1977-86. [PubMed]
- Deshpande CG, Geisinger K, Petersen I, et al. Grading of lung adenocarcinoma: architectural versus nuclear approach. Mod Pathol 2009;22:1596.
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [PubMed]
- Ikeda K, Awai K, Mori T, et al. Differential diagnosis of ground-glass opacity nodules: CT number analysis by three-dimensional computerized quantification. Chest 2007;132:984-90. [PubMed]
- Asamura H, Suzuki K, Watanabe S, et al. A clinicopathological study of resected subcentimeter lung cancers: a favorable prognosis for ground glass opacity lesions. Ann Thorac Surg 2003;76:1016-22. [PubMed]
- Kodama K, Higashiyama M, Yokouchi H, et al. Prognostic value of ground-glass opacity found in small lung adenocarcinoma on high-resolution CT scanning. Lung Cancer 2001;33:17-25. [PubMed]
- Suzuki K, Asamura H, Kusumoto M, et al. “Early” peripheral lung cancer: prognostic significance of ground glass opacity on thin-section computed tomographic scan. Ann Thorac Surg 2002;74:1635-9. [PubMed]
- Yoshida J, Ishii G, Yokose T, et al. Possible delayed cut-end recurrence after limited resection for ground-glass opacity adenocarcinoma, intraoperatively diagnosed as Noguchi type B, in three patients. J Thorac Oncol 2010;5:546-50. [PubMed]
- Xu L, Tavora F, Battafarano R, et al. Adenocarcinomas with prominent lepidic spread: retrospective review applying new classification of the American Thoracic Society. Am J Surg Pathol 2012;36:273-82. [PubMed]
- Xu L, Tavora F, Burke A. ‘Bronchioloalveolar carcinoma’: is the term really dead? A critical review of a new classification system for pulmonary adenocarcinomas. Pathology 2012;44:497-505. [PubMed]
- Kim HK, Choi YS, Kim J, et al. Management of multiple pure ground-glass opacity lesions in patients with bronchioloalveolar carcinoma. J Thorac Oncol 2010;5:206-10. [PubMed]
- Pfannschmidt J, Dienemann H. Surgical treatment of oligometastatic non-small cell lung cancer. Lung Cancer 2010;69:251-8. [PubMed]
- Finley DJ, Yoshizawa A, Travis W, et al. Predictors of outcomes after surgical treatment of synchronous primary lung cancers. J Thorac Oncol 2010;5:197-205. [PubMed]
- Lee HY, Han J, Lee KS, et al. Lung adenocarcinoma as a solitary pulmonary nodule: prognostic determinants of CT, PET, and histopathologic findings. Lung Cancer 2009;66:379-85. [PubMed]