
From pullout-techniques to modular elastic stable chest repair: the evolution of an open technique in the correction of pectus excavatum
Introduction
Pectus excavatum (PE) has been a focus of medicine for many centuries, including investigations into its causes, physical consequences, and treatment options.
When the Basel Professor Johannes Bauhin [1511–1582] presented his son, Johann Bauhin [1541–1612], a 7-year-old boy from the Lower Saxon noble family Derer von Andlau, he subsequently formulated a case report, which he wrote to Johannes Schenck von Grafenberg [1531–1598] in his work “Observationum medicarum, rararum, novarum, admirabiliium et monstrosarum” (“Of rare, new, admirable and astonishing medical observations”) in the section “De partibus vitalibus, thorace contentis” (“On vital structures, contents of the thorax”) under the title “Sterni cum costis ad interna reflexio nativa, spiranda difficultatis causa” (An innate bending of the sternum with ribs - a cause of respiratory problems”) which was published in Freiburg im Breisgau in 1594 (1).
The early reports, as well as those of the 19th century (Table 1), focused on the clinical features and morphology of the deformed chest wall.
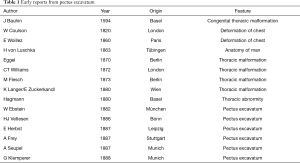
Full table
In contrast, in today’s open society, which is dominated by social networks, the psychosocial weight of this diagnosis (2) is far higher than in the past, leading to an increasing desire for the treatment of patients. The psychological aspects of PE were first mentioned by Charles W. Lester [1949], followed by Paul C. Adkins [1956], Mark M. Ravitch [1962], L. K. Lacquet [1998] and Eckehard Einsiedel [1999] (3-7).
Today, both quantitative indices of deformity and subjective complaints are included when assessing the impairment of a funnel chest (8-10).
When the decision to operate is made, lifestyle considerations are increasingly important, as reflected in the multitude of body image and quality of life evaluations (11-13). Thus, there has been a constant development of efficient surgical methods aimed at achieving anatomical and physiological goals. In addition to correction results and patient acceptance, possible complications (e.g., infections, recurrences, material failure, and life-threatening organ injuries) are of particular interest.
With regard to the actual correction of the thoracic wall, today there are three principally used procedures: cartilage resecting, cartilage-sparing resections, and pure internal bracing of the chest wall without resections.
The second important step is the stabilization of the chest wall, which is performed either by pull-out techniques, by suture and mesh techniques or metallic implants (Figures 1-3). The latter include a variety of metal straps and osteosynthesis implants (Figures 3-6).
The present work considers the historical evolution of the funnel chest correction with focus on open correction procedures in the context of the high treatment demands described above and in the discourse with the minimally invasive repair of pectus excavatum (MIRPE) technique which is clearly accepted as the method of choice for the most of the patients nowadays (25). However, in some scenarios an open procedure can be the appropriate method, for example in a very rigid chest wall, severe asymmetric deformity or demanding redo-procedures, special attention is therefore given to the development of our open operative technique over a 60-year period.
Methods
First, we present the historical development of funnel chest correction, highlighting key publications. Next, we describe the chronological development of our correction method. Treatment results are presented, including complications and comparisons with the findings of other centers. Adjustments of the surgical technique and their respective rational are described. Finally, the rationale of the currently applied correction method is compared to the various correction methods practiced today.
Results
Historical overview
In 1899, Alexander Tietze described the first PE surgery in Wroclaw, where, 6 years later, Sauerbruch (under Johannes Mikulicz-Radecki) developed a vacuum chamber-based thoracic intervention method (14).
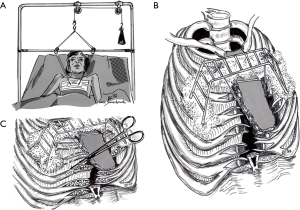
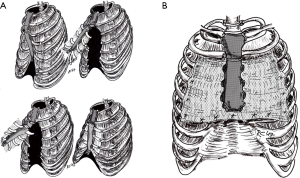
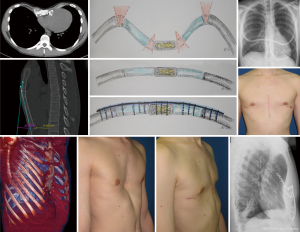
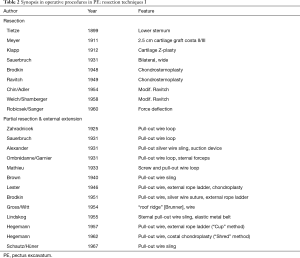
Operative procedures in PE, which were introduced in large numbers at the beginning of the 20th century, were initiated by Ludwig Meyer (1911, Vienna), Rudolf Klapp (1912, Marburg) and Jan Zahradnicek (1925, Prague) with low cartilage resection, or purely sternal extension procedure, with a minimally invasive character (26) (Table 2). On the other hand, the reconstruction of the chest wall after extensive cartilage resection was introduced by Ferdinand Sauerbruch (1931, Berlin; Figure 1A) and Ravitch (1949, Baltimore) (14,27) (Figure 1B,C; Table 2). The highly invasive turn-over principle was described for the first time by Erich Lexer (1927, Freiburg), and continued by Rudolf Nissen (1944, New York), Jean and Robert Judet (1954, Paris), and Juro Wada (1961, Sapporo) (Table 3, Figure 2A).
Full table
Full table
As the extent of resection increased, instability of the anterior chest wall and limitation of respiratory physiology would sometimes develop. To stabilize the chest wall, an external traction device (extension wire dressing) was used in a variety of forms (Table 2, Figure 1). Evidently, in the following decade, infectiological problems in wound healing led to reduced resections and, thus, reduced wound areas, as well as favoring an internal stabilization of the chest wall (3,28) (Table 4).
Full table
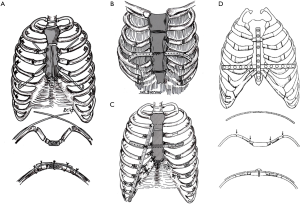
For example, H. Uebermuth from Leipzig reported on 14 patients with a T-shaped sternal osteotomy, chondral wedge resections, and stabilization with crossed Kirschner wires (17) (Figure 3A). Rehbein, on the other hand, stabilized the anterior chest wall with bilaterally, intramedullary-introduced, and sternal supporting rib struts, before various authors described transsternal metal bars (18) (Figure 3).
The application of a plate osteosynthesis was described, as well as the use of a completely metal-free support by a bone chip method (22).
Hartleib’s tibial graft in 1932 showed an alternative with autologous reconstruction in almost 90 years—until today with employment of allogeneic or xenogenic techniques (29). New approaches to correcting the chest wall were introduced by Wickham’s “New Surgery” in 1987, Cuschieri’s “Minimal access surgery” in 1990, and by Nuss in 1998 (30-32). From a biomechanical point-of-view, the spectrum reached from still extensive resections to mobilize the deformed chest wall to completely lossless and resection-free “repositioning” technique by internal bracing using a large-caliber, rigid steel bar (27,32).
Further development of open and closed procedures included modifications aimed at optimizing the mobilization and stabilization of the chest wall. External extension and stress-reducing procedures were employed in the MIRPE, mainly through mini-open approaches, whereas in the conventional open technique, the extensive cartilage resection had been replaced by minimized cartilage resection procedures for reshaping the chest wall (33,34).
This development also marked the adaptation of the proprietary process, whose eventful evolution was achieved through 60 years of clinical experience.
In April 25, 1957, Gerd Hegemann [1912–1999], professor of surgery from 1955 to 1977 in Erlangen, reported in the discussion on “Mediastinum” at the 74th session of the German Society of Surgery in Munich, including ten patients with funnel chest, which he operated in 1956 following the procedures of Mathieu [1933] and Brown [1940] (parasternal resection, sternal loop extension). Following sternum osteotomy in the second intercostal space (ICS), xiphoid resection and subperichondrial cartilage resections (III–VIII) were performed, with the perichondrial tubes separated for complete mobilization of the sternum. Under the sternum, a wire was pulled across, which was finally suspended under tension on a Cup-style dressing (“Körbchen”) with a metal-rope-ladder (Cramer splint) adhered to the skin (19,35) (Figure 1B).
Among 127 patients, there were good cosmetic (38.6%), satisfactory (41.6%), and poor (19.8%) results, with a recurrence rate of 25% (19,36). The extension device, designed for 12 weeks of use, appeared impractical, uncomfortable, and induced infections with relevant sternal wire loop migration. The perichondrial regeneration tissue was not cartilaginous, but fibrous or bony and produced rigidity and unevenness of the chest wall (20). This resulted in the loss of breathing.
Therefore, in 1962, the procedure was modified. The resection of Xiphoid and parasternal cartilage (III–VIII) and the extension principle were retained. However, the perichondrial tubes were filled with finely shredded cartilage and closed by continuous suture (“Shred” method) (19) (Figure 1C).
In 21 patients, chondroplasty increased the substance and stability of the anterior chest wall, but was less successful and gave worse results [more than satisfactory (33.3%), satisfactory (39.4%), and poor (27.3%)] (19). Although the frequency of recurrences was reduced (15%), the rate of wound healing disorders increased significantly (20).
Starting in 1963, a transsternal metal bar implant (“Strut”-method) was used to stabilize the thorax, which was introduced in the 4th ICS and dropped (laterally) onto the ribs. The cartilage resections were minimized and carried out at the ribs on the apex of the deformities, parasternally and anterolaterally, only as narrow wedges excisions (20,36) (Figure 3B).
At first, most out of 66 patients showed the desired cosmetic-functional progress (good 78.9%, satisfactory 18.3%, poor 2.8%) (19), a reduction of the infection (2.9%) and an inpatient discharge after 8 days (20). In 1977, Günter H. Willital (born 1939), who later worked in Munster, Germany, extended the Hegemann method with a wedge-excisions to correct deformed lower rib arches, which he supported with other temple implants in an H-shaped arrangement. Also, a costosternal refixation with sutures was done (21) (Figure 3C). Using this standardized procedure, the complications rate was 5.7% in 1,262 patients, including a recurrence rate of 5% (37,38).
This technique was adopted by Hans P. Hümmer (born 1943) in 1984 and minimized in 2006: intraoperative tensiometry was used to identify the retinal forces of the sternum during fractionated dissection of the anterior chest wall, thus allowing an individually adapted procedure with a considerably reduced length of the surgical approach (34).
In 2010, the method was significantly modified by the authors to achieve a tension-free “anatomization” of the deformed chest wall. Because of the high rate of painful relapses of deformity, bar dislocations, costosternal dislocations (“stairway-phenomenon”) with step formation, and an increased rate of wound healing disorders, the procedure was again based on the Willital-Hegemann technique (23). We focused on addressing all of the deformed cartilage and bone areas of the anterior chest wall. For women, this novel surgical approach provided a sufficiently long submammary incision and, for men, a classical median sternal approach, and possibly additional (limited) incisions over deformed ribs of the lower costal margin. These were corrected by a costo-chondroplasty using the technique of Jean-Maria Wihlm and adapted by suturing, while the peristernal chest wall was again stabilized by 1–2 transsternal bars (39).
The treatment of recurrent deformities with unstable sternal ribs was a particular challenge facing surgeons at that time was. These were then stabilized in the context of the recurrence correction in addition to the bar by means of costosternal, locked plate osteosynthesis, and brought to healing (23).
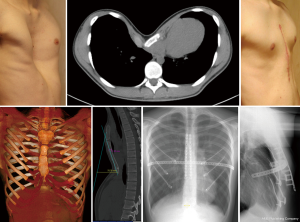
Due to the biomechanical superiority of the locked plate osteosynthesis, a long version of the low-profile plates was developed, which allowed the entire anterior chest wall to be attached from rib-to-sternum-to-rib, including all of their correction segments. This innovative procedure was initially used in case of redo surgery and was inaugurated as elastic-stable-chest-repair (24) (Figure 4).
As early as 2011, high patient comfort and anatomical remodeling of the corrected chest wall achieved good results, even with primary corrections on adult patients. The thoracic wall, mobilized by minimized osteo- and chondrotomies, was left en bloc, respecting the anatomy, and replaced by the above mentioned stabilized costo-sternocostal plate osteosynthesis.
An additional longitudinal plate osteosynthesis of the transverse sternum osteotomy was shown to be advantageous in terms of primary sternum stability and was associated with a significant reduction of pain postoperatively and a quasi-perfect remodeling of the sternum to the normal form.
Following the widely used example of orthopedic axis corrections of long bones, we complemented the procedure with a precise preoperative planning of the resection based on CT measurements and made accurate saw cuts on the sternum and ribs using specially developed sawing jaws. By this approach, we were able to achieve a highly accurate correction of the chest wall.
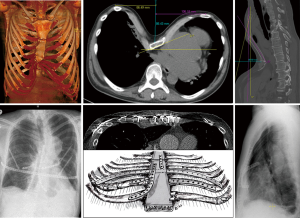
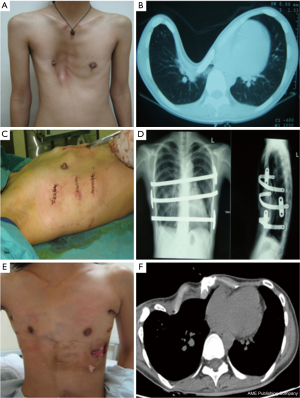
The challenge was now to master the correction of particularly demanding deformities—such as wide-base funnels with the involvement of bony rib deformities and strongly asymmetrical shapes with bony, sternal rotation, and often deep parasternal rib valleys (Figures 5,6).
While the bar dynamically supports the peristernal chest wall, a frequently multi-layered, de-rotatory sternotomy is supported under longitudinal plates, and multiple incisions mobilized rib valleys under transversely applied plates. In the case of a rib osteotomy with a laterally located apex of curvature, the latter is either primarily flattened or stabilized by an intramedullary splint to provide a reliable abutment against the resting metal bar and at the same time allow anatomical remodeling of the stabilized rib osteotomy (Figures 5,6).
Lower costal margin correction is also stabilized by a locked plate osteosynthesis, incorporating the cartilage portions according to the above method, which drastically reduced the complication rate with painful instability and exostosis in favor of patient satisfaction (23,24).
ESCR was performed on 86 patients [39 (45.3%) primary and 47 (54.7%) recurrence] between 2011 and 2015. Of the 47 recurrence patients, 17 were operated following MIRPE the remainder after an open procedure (63.8%). Pain was the indication for reoperation in 38 patients (80.9%), of whom 24 had pseudarthrosis (63.2%). Primary corrections were performed in 77% as a hybrid technique and recurrence corrections in 34%. The lower costal margins were corrected primarily in 41% of the patients, in relapses at least in 31.9% out of the cases.
In addition to the plannable, precise en bloc mobilization of the chest wall, the described techniques allow for their modular stabilization through innovative implant combinations with the goal of definitive osteo-and chondral remodeling.
Thus, only less than 5% wound healing disturbances were observed in the follow-up date, and three patients were revised again. In two cases, we detected a renewed growth at the age of 18 and 19 years, with the consequence of a recurrence and plate break. Another case required bone augmentation following a previous 8-fold correction and connective rib and sternum necrosis in low-grade infection. This patient also healed without irritation and with an excellent cosmetic result.
Moreover, 38,4% of the patients requested material removal (64.1% primary, 17.0% recurrence) as part of another operation.
Discussion
Traditionally, PE correction has been a mostly rigorous, resecting procedure, focusing on the deformation of the chest wall, disregarding physiological-anatomical aspects and accepting severe consequences in thoracic instability. Due to the initially favored, external stabilization of the chest wall by lengthy extension and traction techniques, this approach was associated with a danger of infection—nevertheless, such techniques were used for more than half a century until the 1970s. At the same time, various methods of internal stabilization were carried out for almost 90 years, none of which became universally accepted. Despite the propensity for resection, internal stabilization was essential for minimizing major complications, such as instability and infection. The procedure of Hegemann, first presented in 1957 (35), exemplifies the change in the paradigm of thoracic functionality and its stabilization; the common parasternal resection of cartilage was always done with the protection of the perichondrium tubes, which are highly potent for regeneration of cartilage tissue. However, ultimately, only fibrous or osseous regenerates were detected, which not only worsened the compliance of the anterior chest wall but also promoted recurrence through anatomical unevenness and lack of arching (19,35,36).
After achieving just 38.6% positive results (19) in the first series, an improvement was achieved by introducing a corrected, anatomical structure on the anterior chest wall: resected cartilage tracts were transformed by “chipping” into shapeable biomass (“shreds”) and sewn into the perichondrial tubes (Figure 1C). However, the vitalization of this material was eliminated. Thus, the results worsened further and aggravated the wound healing problem due to the necrosis aspect (19,20,36). Finally, after 7 more years, the avoidance of the resection method with external stabilization changed to an internal fixation, which has elsewhere already been carried out in 1959 using transsternal transverse bars by Sulamaa and Wallgren or 1961 by Adkins. By minimizing the resection to small, wedge-shaped withdrawals and the use of a transsternal bar, 78.9% good results could be achieved (19); 10 years after the first interventions a reliable procedure had been established. Ten years later, Willital turned to a large-scale correction of the anterior chest wall and included the costal arches in Hegemann’s reconstruction concept (21) (Figure 3C). The resulting “H” construction of the bars has even been used in recent years (37).
The costosternal approximation using sutures, which was also introduced by Willital, opened in 2006 after the “minimization” of the procedure by Hümmer (34) an evident pain problem by costosternal dislocations with the formation of mediastinal callus formations due to sternocostal pseudarthrosis, which has been described perfectly with “stairway-phenomenon” (23,24). By minimized access and purely sternal focused mobilization, it was necessary to assume a disbalance of anatomical restoring forces and costosternal fixation support, especially of the anterolaterally deformed ribs. Therefore, after analyzing the open, minimized procedure, special emphasis was again placed on a complex but lossless mobilization of the anterior chest wall by parasternal, costomedial, and costolateral open or closed wedge scoring, which was validated by appropriate preoperative CT planning. Likewise, the unsafe costosternal fixation technique with threads was replaced by a second biomechanical principle of locked plating. Using variable locked plates, a “dome”-shaped reconstruction of the peristernal chest wall could be achieved by the lifting of the sternum (Figure 4).
The sternum acts as a “capstone” of an arch-like construction to stably unite the implants, acting as elastic arch wires (the buttress arch). After analysis of the implants used, it was shown that in the context of the first correction different biomechanical requirements exist than with recurrent interventions. Therefore, in the majority of the patients with thoracic pain syndromes with instability due to pseudarthrosis, only 34% (vs. 77% primary correction) required additional sternal support with a bar, whereas correction of sternal malformations (e.g., angulation and torsion) was far more common in primary correction with longitudinal plates (61.5% vs. 29.8%; Figure 5). In the costosternal area, there was also a dominance of plate used in recurrence (74.4% vs. 56.4%), and, moreover, plates had been mostly used for long-distance bridging costo-sternocostal (55.3% vs. 18%, Figure 4). This shows the high variability and practicability of the ESCR system, even in complex thoracic malformations (Figure 6).
Significant need for correction, which in addition to the crests of deformed lower ribs also remedies apparent cosmetic losses, exists on the lower costal margin. There are no minimally invasive alternatives so that an open procedure must shape and stabilize the sometimes-complex malformations on the costal arch itself, but also on the feeding ribs. Correspondingly, the most bilateral approach (41.0% vs. 31.9%) dominates the first correction, often supplemented by additional corrections of individual lateral ribs (41.0% vs. 8.5%).
The biomechanical principle of lifting the mobilized, possibly also plate-corrected sternum, with the aid of the transverse transsternal bar and the stabilization of the entire peristernal area using locked plate osteosynthesis shows a high potency for “anatomizing” the deformed chest wall. After fusion and consolidation, the elastic bar can be removed after 1 year, through a small lateral incision, leaving the inert, anatomically shaped, low-profile implants in situ. The described method provides a high precision of correction as the CT planned en bloc mobilized chest wall deformity by minimized chondro- and osteotomies technique described here.
It allows the modular corrections in the manner shown with the result of an anatomical remodeling of the chest wall. By this approach can the recurrence of the deformity and pseudarthroses, as well as persisting instability, be permanently avoided.
The multiple applied MIRPE, however, can achieve this remodeling best in the still elastic, growing, juvenile chest wall. However, it is more and more applied in adults as well but it is not a successful therapy of sternocostal dislocations or rigid deformities (Figure 7) (40,41).
Conclusions
Patients with chest wall deformities show a high demand in lifestyle and request of surgical techniques. The minimally invasive technique developed by Nuss performs best for symmetrical deformities of the elastic, growing skeleton, whereas adult patients require open surgery in severe cases. These more invasive open procedures, such as ESCR, can be considered for example in the following: asymmetric deformities, multidimensional sternum deformity, recurrence deficiency with instability or resection-related deformities, and deformity of the lower rib arch (costal flaring).
We propose that a mobilization of the chest wall using limited cartilage and bone incisions should be possible without losses, as opposed to cartilage-resecting procedures. Modular stabilization using stable locked plate osteosynthesis combines the advantages of anatomical remodeling with optimal stability.
Acknowledgments
This manuscript is dedicated to all those involved in the 60-year development of funnel chest surgery at the University Hospital Erlangen.
Footnote
Conflicts of Interest: S Schulz-Drost is a member of the AO Thoracic Expert Group (THEG) and has a consultant agreement with DePuySynthes. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study respects the rules of the local ethical committee. The institutional ethical committee has not been involved since no personal data had been used for the study and no investigations on humans had been done.
References
- Bauhinus J. Observatio CCLXIV. In: Schenck von Grafenberg J. editor. Observationum medicarum, rararum, novarum, admirabilium et monstrosarum. Liber secundus. De partibus vitalibus, thorace contentis. Excudebat Martinus Becklerus. Band II, Martinus Becklerus, Friburgi Brisgoiae, 1594.
- Joraschky P, Loew T, Röhricht F. Körpererleben und Körperbild: Ein Handbuch zur Diagnostik. Stuttgart: Schattauer, 2008.
- Lester CW. Funnel chest and allied defomities of the thoracic cage. J Thorac Surg 1949;19:507-22.
- Adkins PC, Gwathmey O. Pectus excavatum: an appraisal of surgical treatment. J Thorac Surg 1958;36:714-24. [PubMed]
- Ravitch MM. The chest wall. In: Benson CD, Mustard T, Ravitch MM, et al. editors. Pediatric Surgery. Chicago: Year Book Medical Publishers, 1962:238
- Lacquet LK, Morshuis WJ, Folgering HT. Long-term results after correction of anterior chest wall deformities. J Cardiovasc Surg (Torino) 1998;39:683-8. [PubMed]
- Einsiedel E, Clausner A. Funnel chest. Psychological and psychosomatic aspects in children, youngsters, and young adults. J Cardiovasc Surg (Torino) 1999;40:733-6. [PubMed]
- Haller JA Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904-6. [Crossref] [PubMed]
- St Peter SD, Juang D, Garey CL, et al. A novel measure for pectus excavatum: the correction index. J Pediatr Surg 2011;46:2270-3. [Crossref] [PubMed]
- Ewert F, Syed J, Kern S, Besendörfer M, et al. Symptoms in Pectus Deformities: A Scoring System for Subjective Physical Complaints. Thorac Cardiovasc Surg 2017;65:43-9. [Crossref] [PubMed]
- Kelly RE Jr, Cash TF, Shamberger RC, et al. Surgical repair of pectus excavatum markedly improves body image and perceived ability for physical activity: multicenter study. Pediatrics 2008;122:1218-22. [Crossref] [PubMed]
- Krille S, Müller A, Steinmann C, et al. Self- and social perception of physical appearance in chest wall deformity. Body Image 2012;9:246-52. [Crossref] [PubMed]
- Kelly RE Jr, Lombardo ML. Psychologic effects, body image and pectus excavatum and carinatum. In: Saxena AK. editor. Chest Wall Deformities. Berlin: Springer, 2017:169-74.
- Sauerbruch F. Operative Beseitigung der angeborenen Trichterbrust. Deutsche Zeitschrift f. Chirurgie 1931;234:760-4.
- Wada J. Surgical correction of the funnel chest "sternoturnover". West J Surg Obstet Gynecol 1961;69:358-61. [PubMed]
- Robicsek F, Fokin A. Surgical correction of pectus excavatum and carinatum. J Cardiovasc Surg (Torino) 1999;40:725-31. [PubMed]
- Uebermuth H. Erfahrungen zur Operation der Trichterbrust. Langenbecks Arch klin Chir 1957;287:234-8. [Crossref] [PubMed]
- Rehbein F, Wernicke HH. The operative treatment of the funnel chest. Arch Dis Child 1957;32:5-8. [Crossref] [PubMed]
- Hegemann G. Kosmetische und funktionelle Ergebnisse operativer Massnahmen bei Trichterbrust. Langenbecks Arch Klin Chir 1967;319:526-36. [Crossref]
- Gall FP, Hegemann G, Kollermann MW, et al. Surgical treatment of funnel chest. Dis Chest 1967;52:10-4. [Crossref] [PubMed]
- Willital GH. Proceedings: Treatment of funnel chest. Surgical indication, time and method. MMW Munch Med Wochenschr 1975;117:367-8. [PubMed]
- Gotzen L, Dragojevic D. Funnel chest correction by use of AO implants and instruments. Thorac Cardiovasc Surg 1979;27:61-4. [Crossref] [PubMed]
- Schulz-Drost S, Syed J, Besendörfer M, et al. Sternocostal dislocation following open correction of pectus excavatum-"stairway phenomenon": complication management by means of sternocostal locking titanium plate osteosynthesis. Thorac Cardiovasc Surg 2014;62:245-52. [PubMed]
- Schulz-Drost S, Syed J, Besendörfer M, et al. Elastic stable chest repair as a means of stabilizing the anterior chest wall in recurrent pectus excavatum with sternocostal pseudarthrosis: An innovative fixation device. Thorac Cardiovasc Surg 2015;63:419-26. [PubMed]
- Pilegaard HK. Short Nuss bar procedure. Ann Cardiothorac Surg 2016;5:513-8. [Crossref] [PubMed]
- Meyer L. Zur Chirurgischen Behandlung der angeborenen Trichterbrust. Verhandlungen der Berliner Medizinischen Gesellschaft 1911;42:364-73.
- Ravitch MM. The Operative Treatment of Pectus Excavatum. Ann Surg 1949;129:429-44. [Crossref] [PubMed]
- Fonkalsrud EW. Open repair of pectus excavatum with minimal cartilage resection. Ann Surg 2004;240:231-5. [Crossref] [PubMed]
- Muschik M, Wagenitz A, Zippel H. Surgical treatment of pectus carinatum with the modified Ravitch method. Z Herz-, Thorax- Gefäßchir 1998;12:226.
- Wickham JE. The new surgery. Br Med J (Clin Res Ed) 1987;295:1581-2. [Crossref] [PubMed]
- Cuschieri A. Minimal access surgery: the birth of a new era. J R Coll Surg Edinb 1990;35:345-7. [PubMed]
- Nuss D, Kelly RE Jr, Croitoru DP, et al. A 10-year review of a minimally invasive technique for the correction of pectus excavatum. J Pediatr Surg 1998;33:545-52. [Crossref] [PubMed]
- Park HJ, Jeong JY, Jo WM, et al. Minimally invasive repair of pectus excavatum: a novel morphology-tailored, patient-specific approach. J Thorac Cardiovasc Surg 2010;139:379-86. [Crossref] [PubMed]
- Weber PG, Hümmer HP. The "new" Erlangen technique of funnel chest correction - minimalization of a well working procedure. Zentralbl Chir 2006;131:493-8. [Crossref] [PubMed]
- Hegemann G. Aussprache Mediastinum, Vortrag Übermuth. Langenbecks Arch Klin Chir u. Dtsch Z Chir 1957;287:244.
- Schoberth H. Die Trichterbrust. In: Bauer KH, Brunner A, Lindemann K. editors. Ergeb Chir Orthop. Berlin: Springer, 1961;43:122-202.
- Saxena AK, Willital GH. Valuable lessons from two decades of pectus repair with the Willital-Hegemann procedure. J Thorac Cardiovasc Surg 2007;134:871-6. [Crossref] [PubMed]
- Saxena AK. Overview of repair of pectus excavatum type of deformities. In: Saxena AK. editor. Chest Wall Deformities. Berlin: Springer, 2017:329-49.
- Brochhausen C, Turial S, Müller FK, et al. Pectus excavatum: history, hypotheses and treatment options. Interact Cardiovasc Thorac Surg 2012;14:801-6. [Crossref] [PubMed]
- Zhang DK, Tang JM, Ben XS, et al. Surgical correction of 639 pectus excavatum cases via the Nuss procedure. J Thorac Dis 2015;7:1595-605. [PubMed]
- Pilegaard HK. Extending the Nuss procedure in patients older than 30 years. Eur J Cardiothorac surg 2011;40:334-7. [PubMed]


