Quantifying the expression of tumor marker genes in lung squamous cell cancer with RNA sequencing
Introduction
Lung cancer is the most commonly diagnosed cancer as well as the leading cause of cancer-related death. This disease accounted for 13% (1.6 million) of total cancer cases and 18% (1.4 million) of deaths caused by cancer in 2008 (1). Squamous-cell lung cancer, one of the most common types of lung cancer, typically originates in the large airways and represents approximately 20-30% of lung cancer cases in recent years (2). The overall survival of early stage patients is quite good, but most patients are diagnosed at an advanced stage when there are fewer curative opportunities. Therefore, early diagnosis is the key to improve patient outcomes for squamous-cell lung cancer.
Tumor markers have been widely applied in cancer diagnosis over the last two decades due to convenience, low cost, reproducibility and non-invasiveness. Most previous studies have focused on tumor marker levels in the serum. Here, we studied the expression of several commonly used tumor-marker genes in tumor and normal tissues. Squamous-cell lung cancer and adenocarcinoma are so different from each other (3). Therefore, we measured the expression of some commonly used tumor markers in squamous-cell lung cancer tissues using RNA sequencing (RNA-Seq) to screen for genes that may be suitable tumor markers in squamous cell lung cancer. We then investigated possible correlations between these tumor markers and clinical characteristics.
Materials and methods
Ethics statement
This study was approved by the Ethics Committee of Zhongshan Hospital, Fudan University {Approved No. 2011-219[2]}. Written informed consent was obtained from each patient participating in this study.
Tissue samples and clinicopathological characteristics
Samples were obtained from patients with squamous-cell lung cancer who underwent surgical resection between September and December, 2012 at Zhongshan Hospital, Fudan University, Shanghai, China. Clinicopathological characteristics were recorded for all patients. Normal lung specimens were resected at least 3 centimeters away from the tumor margin, while tumor samples were carefully extracted from the center of squamous-cell carcinoma. All samples were flash frozen in liquid nitrogen after removal and then saved at –80 °C. Part of each sample was paraffin embedded, HE stained and checked by an experienced pathologist to make ensure that no cancer cell existed in the normal tissues and more than 80% of cells in every tumor sample were squamous carcinoma. Finally, five matched pairs of normal tissue samples and lung squamous-cell carcinoma tissues and another 39 lung squamous-cell carcinoma tissues were obtained. These samples were then used in RNA-Seq.
RNA preparation
Total RNA from each sample was extracted with Trizol (Invitrogen, Carlsbad, CA, USA), re-dissolved in DEPC-treated water and quantified using NanoVue Plus spectrophotometry (GE Healthcare, Fairfield, CT, USA). RNA integrity was evaluated using agarose gel electrophoresis, and DNA contamination was eliminated using gDNA Eraser (Takara, Tokyo, Japan) according to the manufacturer’s guidelines.
RNA sequencing (RNA-Seq)
The mRNA component of total RNA was converted into a library of template molecules suitable for sequencing using TruSeq®RNA Sample Preparation Kit v2 (Illumina, SanDiego, CA, USA) according to the manufacturer’s guidelines. mRNA was purified and fragmented and first and second strand cDNA was synthesized. RNA was then subjected to end repair, 3' end adenylation, ligation of adapters, PCR amplification of cDNA libraries procedure, among others. Sequencing was then performed using a Genome Analyzer II (Illumina, SanDiego, CA, USA) according the manufacturer’s recommendation. Sequence analysis was performed using Galaxy software (http://galaxyproject.org) to calculate the reads per kilobase of exon model per million mapped reads (RPKM) of every transcript. Then the RPKM of all transcripts from each gene were added up to evaluate that gene’s expression. Each sample was sequenced twice and the average of the RPKM value of each gene was adopted to reflect its expression level.
Statistical analysis
The RPKM data derived from RNA-Seq were analyzed using SPSS for windows, version 20 (IBM, Armonk, NY, USA). The mean of the RPKM was used to evaluate the gene expression level. The expression profiles of tumor maker genes in normal samples were compared with paired tumor samples using the paired t-test and t-test for comparing genes’ expressions between normal and all tumor samples. Spearman correlation analysis was used to identify correlations between the expression of tumor marker gene expression and clinicopathological characteristics, and Pearson correlation analysis was used to identify correlations between CA125, CYFRA21-1, NSE and SCC.
Results
Patient clinicopathological characteristics
The clinicopathological characteristics of all 44 patients are listed in Table 1. All of the patients in our study were male, because most squamous-cell lung cancers occur in older male patients. Female patients accounted for only 7.8% (104/1,320) of squamous-cell lung cancer in our department from 2005-1-1 to 2011-12-31. In our study, none of the patients were at N3 or M1 stage, because these patients were not suitable for radical surgical resection. No patients were in stage IV for the same reason.
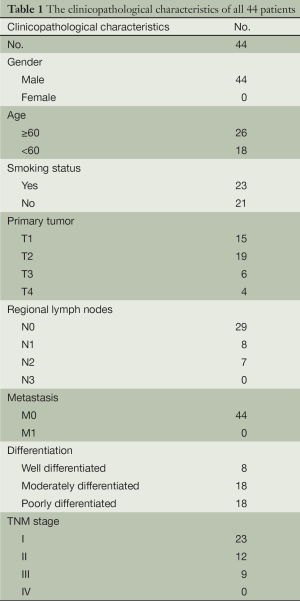
Full table
Expression profiles of commonly used tumor markers
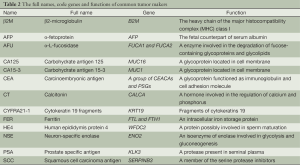
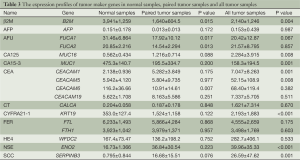
We evaluated 13 commonly used tumor markers, including β2M, AFP, AFU, CA125, CA15-3, CEA, CT, CYFRA21-1, FER, HE4, NSE, PSA and SCC. Their full names, encoding genes and functions are presented in Table 2. We listed all encoding genes and expression profiles for tumor markers that are encoded by more than one gene, with the exception of CEA. CEA is encoded by a group of genes, including at least 12 carcinoembryonic antigen-related cell adhesion molecule genes and 11 pregnancy-specific beta-1-glycoprotein genes, so we listed the four most highly expressed genes. The expression of PSA was too low to be detected in most of our samples, so it is not listed in Table 3.
Full table
Full table
The expression of CA125, CYFRA21-1, NSE and SCC increased in tumor samples, and there was statistical significance in the difference between squamous cell lung cancer and normal tissues for these genes. While CEA was produced by the co-expression of multiple genes, only expression from some of these genes produced statistical significance. The expression of β2M and CA15-3 was lower in squamous cell carcinoma relative to normal tissues, and there was statistical significance in these differences. There is no statistical significance in the expression of the other tumor markers tested, including AFP, AFU, CT, FER and HE4 (Table 3).
Correlation analyses between the expression of four tumor marker genes and patient clinicopathological characteristics
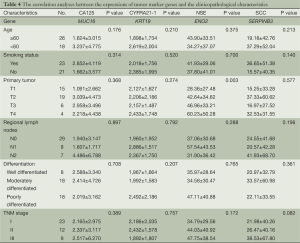
We calculated the correlation between the expression of four tumor marker genes (CA125, CYFRA21-1, NSE and SCC) and patient clinicopathological characteristics, including age, smoking status, size of primary tumor, condition of regional lymph nodes, differentiation and TNM stage. Statistical significance only existed for differences in NSE across different T stages.
The expression levels of NSE and SCC tended to increase with increasing TNM stage, but changes did not reach statistical significance (Table 4). Increasing sample numbers may achieve statistical significance, however. There were no obvious correlations in the expression profiles of CA125, CYFRA21-1, NSE and SCC (Table 5).
Full table
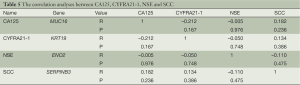
Full table
Discussion
Lung cancer is the leading cancer site in males and accounts for 11% of total female cancer deaths in developing countries (1). Squamous-cell lung cancer is one of the most common subtypes of lung cancer. Most squamous-cell lung cancer occurs in male patients who smoke, and this disease is often diagnosed at an advanced and inoperable stage (4). Although considerable progress has been made in the early diagnosis and treatment of lung cancer, outcomes are typically not satisfactory. Because most squamous-cell lung cancers originate in the main bronchus, they are difficult to detect with imageological examination. The evaluation of serum tumor markers could provide an important supplementary examination method for diagnosis when disease manifestations are not obvious and imageological examination is negative (5,6). Therefore, there is significant clinical significance for the research of serum tumor markers in squamous cell lung cancer. An elevated level of serum tumor markers is typically caused by the level of these tumor markers in tumor tissues. In this study we performed RNA-Seq on five matched pairs of normal and squamous-cell lung cancer tissues and another 39 squamous-cell lung cancer tissues. Thirteen commonly used tumor marker genes were tested to screen for appropriate tumor markers. Finally, the genes encoding CA125, CYFRA21-1, NSE and SCC were shown to be expressed at higher levels in tumor than in normal tissues. Therefore, they might be suitable as tumor markers for screen squamous-cell lung cancer.
SCC has been implicated in tumor growth, and it also inhibits the apoptosis of tumor cells (7,8). As a commonly used tumor marker, SCC is valuable for the detection of many types of squamous cell carcinoma, including esophageal, head and neck and lung squamous cell carcinoma (9,10). SCC resides in the cytosol of squamous cells and is released into the circulation during squamous cell carcinoma (11). NSE is widely used in the screening in small cell lung cancer (12-14), but its function in squamous cell lung cancer has not been clearly studied. NSE as an enzyme active in glycolysis, and the rate of glycolysis is extremely elevated in tumor proliferation, a phenomenon called the “Warburg effect” (15). We also detected that the level of NSE was significantly higher in tumors than in normal tissues. CYFRA 21-1 is the serum dissolution fragment of cytokeratin 19, which is expressed exclusively in epithelial cells and tumors of epithelial origin (16). Previous studies have suggested that CK 19 played a part in the aggressive behavior of tumor cells and was connected with the differentiation and invasion of tumor cells (17). Increased serum CYFRA 21-1 is the result not only of cytokeratin release as a consequence of cell lysis or necrosis, but also of the degradation of cytokeratin filaments by activated proteases in tumor cells (18,19). The high level of CYFRA21-1 in patients with squamous cell lung cancer makes this protein the most sensitive of all of the currently studied tumor markers (20). CA125 is also found at a high level in ovarian carcinomas and lung cancer (21,22). Although previous studies have suggested that tumor markers such as CT, Ferritin and HE4 were present at high levels in lung cancer (23-25), they were not found to be elevated in the tumor tissues from our study.
Most previous studies have focused on the detection of tumor markers in the serum. However, few studies have investigated tumor markers in tissue and, in particular, the expression of their encoding genes. To address this shortcoming, we measured the expression of tumor markers in tissues in our study. In the 13 tumor markers we tested, CA125, CYFRA21-1, NSE and SCC have been commonly used in clinical practice for diagnosis or prognosis of NSCLC (21,26-28). These tumor markers also express at a higher level in tumor samples than in normal samples in our study. Our study also supports their use in clinical practice. Several studies have demonstrated the use of these tumor markers in TNM staging (29-31). We also evaluated any correlations between the expression of the encoding genes of these tumor markers and patient clinicopathological characteristics, but we identified no statistically significant differences. We evaluated whether serum levels of these tumor markers would increase with increasing tumor volume, this being the reason why serum levels for these tumor markers can reflect TNM stages. The outcome of RNA-seq is an expression of unit volume, however, so it may not change with tumor growth.
Squamous cell lung cancer and adenocarcinoma are the two most common histologic subtypes of non-small cell lung cancer. However, these two subtypes are quite different in host susceptibility, clonal evolution, molecular evolution and molecular profiling (3). Previous studies have suggested that the elevated serum SCC percentage is highest in squamous-cell lung cancer, while this percentage is substantially lower in other types (32). NSE expression is higher in squamous-cell lung cancer with neuroendocrine differentiation. The serum CYFRA 21-1 level has been shown to be particularly elevated in squamous cell cancers (28). CA125 has been shown to be substantially expressed in large cell carcinoma and adenocarcinoma, but this protein could not be detected in a squamous lung cell line (33). Therefore, it is not clear whether or not our study can be directly applied to other types of lung cancer, such as adenocarcinoma or large cell lung cancer.
RNA-Seq has emerged as a popular high-throughput technology in recent years. In this technique, transcript levels are quantified in RPKM, which reflects the molar concentration of a transcript normalized by the total read number of the measurement. This normalization avoids common experimental deviations and also facilitates comparisons between multiple genes and samples. As such, RNA-Seq is an ideal method for global gene expression analysis.
Conclusions
Encoding-gene expression for CA125, CYFRA21-1, NSE and SCC was elevating in tumor tissues of squamous-cell lung cancer, while β2M and CA15-3 were expressed at a lower level in squamous cell lung cancer tissues. PSA could not be detected in most samples, and there was no significant difference between tumor and normal samples for the others tumor markers evaluated in this study, AFP, AFU, CEA, CT, FER and HE4.
Acknowledgements
Funding: This work was supported by Doctoral Program Foundation of Institutions of Higher Education of China (No. 20110071120066).
Authors’ contribution: Qun Wang supervised this study, designed experiments and edited the paper with Yu Shi. Lin Wang, Cheng Zhan and Yongxing Zhang designed and carried out experiments, collected and analyzed data and co-wrote the paper. Jun Ma collected tissues and clinical characteristics of the patients with Junjie Xi and Wei Jiang, they also performed experiments with Yu Shi.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Travis WD. Pathology of lung cancer. Clin Chest Med 2011;32:669-92. [PubMed]
- Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med 2008;359:1367-80. [PubMed]
- Jemal A, Center MM, DeSantis C, et al. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 2010;19:1893-907. [PubMed]
- Ather MH, Abbas F, Faruqui N, et al. Correlation of three immunohistochemically detected markers of neuroendocrine differentiation with clinical predictors of disease progression in prostate cancer. BMC Urol 2008;8:21. [PubMed]
- Hinestrosa MC, Dickersin K, Klein P, et al. Shaping the future of biomarker research in breast cancer to ensure clinical relevance. Nat Rev Cancer 2007;7:309-15. [PubMed]
- Kato H. Expression and function of squamous cell carcinoma antigen. Anticancer Res 1996;16:2149-53. [PubMed]
- Suminami Y, Nawata S, Kato H. Biological role of SCC antigen. Tumour Biol 1998;19:488-93. [PubMed]
- Mino N, Iio A, Hamamoto K. Availability of tumor-antigen 4 as a marker of squamous cell carcinoma of the lung and other organs. Cancer 1988;62:730-4. [PubMed]
- Palermo F, Carniato A, Fede A, et al. Serum SCC-Ag in head and neck squamous cell carcinoma. Int J Biol Markers 1990;5:118-20. [PubMed]
- Uemura Y, Pak SC, Luke C, et al. Circulating serpin tumor markers SCCA1 and SCCA2 are not actively secreted but reside in the cytosol of squamous carcinoma cells. Int J Cancer 2000;89:368-77. [PubMed]
- Satoh H, Ishikawa H, Kurishima K, et al. Cut-off levels of NSE to differentiate SCLC from NSCLC. Oncol Rep 2002;9:581-3. [PubMed]
- Schneider J, Velcovsky HG, Morr H, et al. Comparison of the tumor markers tumor M2-PK, CEA, CYFRA 21-1, NSE and SCC in the diagnosis of lung cancer. Anticancer Res 2000;20:5053-8. [PubMed]
- Molina R, Auge JM, Escudero JM, et al. Mucins CA 125, CA 19.9, CA 15.3 and TAG-72.3 as tumor markers in patients with lung cancer: comparison with CYFRA 21-1, CEA, SCC and NSE. Tumour Biol 2008;29:371-80. [PubMed]
- Warburg O, Wind F, Negelein E. The metabolism of tumors in the body. J Gen Physiol 1927;8:519-30. [PubMed]
- Moll R, Krepler R, Franke WW. Complex cytokeratin polypeptide patterns observed in certain human carcinomas. Differentiation 1983;23:256-69. [PubMed]
- Isic Dencic T, Cvejic D, Paunovic I, et al. Cytokeratin19 expression discriminates papillary thyroid carcinoma from other thyroid lesions and predicts its aggressive behavior. Med Oncol 2013;30:362. [PubMed]
- Kulpa J, Wójcik E, Reinfuss M, et al. Carcinoembryonic antigen, squamous cell carcinoma antigen, CYFRA 21-1, and neuron-specific enolase in squamous cell lung cancer patients. Clin Chem 2002;48:1931-7. [PubMed]
- Sheard MA, Vojtesek B, Simickova M, et al. Release of cytokeratin-18 and -19 fragments (TPS and CYFRA 21-1) into the extracellular space during apoptosis. J Cell Biochem 2002;85:670-7. [PubMed]
- Takei Y, Minato K, Tsuchiya S, et al. CYFRA 21-1: an indicator of survival and therapeutic effect in lung cancer. Oncology 1997;54:43-7. [PubMed]
- Kimura Y, Fujii T, Hamamoto K, et al. Serum CA125 level is a good prognostic indicator in lung cancer. Br J Cancer 1990;62:676-8. [PubMed]
- Dietel M, Arps H, Klapdor R, et al. Antigen detection by the monoclonal antibodies CA 19-9 and CA 125 in normal and tumor tissue and patients’ sera. J Cancer Res Clin Oncol 1986;111:257-65. [PubMed]
- McKenzie CG, Evans IM, Hillyard CJ, et al. Biochemical markers in bronchial carcinoma. Br J Cancer 1977;36:700-7. [PubMed]
- Volpino P, Cangemi V, Caputo V, et al. Clinical usefulness of serum ferritin measurements in lung cancer patients. J Nucl Med Allied Sci 1984;28:27-30. [PubMed]
- Galgano MT, Hampton GM, Frierson HF Jr. Comprehensive analysis of HE4 expression in normal and malignant human tissues. Mod Pathol 2006;19:847-53. [PubMed]
- Vassilakopoulos T, Troupis T, Sotiropoulou C, et al. Diagnostic and prognostic significance of squamous cell carcinoma antigen in non-small cell lung cancer. Lung Cancer 2001;32:137-44. [PubMed]
- Ferrigno D, Buccheri G, Giordano C. Neuron-specific enolase is an effective tumour marker in non-small cell lung cancer (NSCLC). Lung Cancer 2003;41:311-20. [PubMed]
- Stieber P, Hasholzner U, Bodenmüller H, et al. CYFRA 21-1. A new marker in lung cancer. Cancer 1993;72:707-13. [PubMed]
- Molina R, Filella X, Augé JM, et al. Tumor markers (CEA, CA 125, CYFRA 21-1, SCC and NSE) in patients with non-small cell lung cancer as an aid in histological diagnosis and prognosis. Comparison with the main clinical and pathological prognostic factors. Tumour Biol 2003;24:209-18. [PubMed]
- Diez M, Gomez A, Hernando F, et al. Serum CEA, CA125, and SCC antigens and tumor recurrence in resectable non-small cell lung cancer. Int J Biol Markers 1995;10:5-10. [PubMed]
- Foa P, Fornier M, Miceli R, et al. Tumour markers CEA, NSE, SCC, TPA and CYFRA 21.1 in resectable non-small cell lung cancer. Anticancer Res 1999;19:3613-8. [PubMed]
- Sánchez De Cos J, Masa F, de la Cruz JL, et al. Squamous cell carcinoma antigen (SCC Ag) in the diagnosis and prognosis of lung cancer. Chest 1994;105:773-6. [PubMed]
- Homma S, Satoh H, Kagohashi K, et al. Production of CA125 by human lung cancer cell lines. Clin Exp Med 2004;4:139-41. [PubMed]


