Pulmonary rehabilitation for COPD: are programs with minimal exercise equipment effective?
Introduction
Pulmonary rehabilitation is an essential component of chronic obstructive pulmonary disease (COPD) management with strong evidence supporting its efficacy (1). Clinically significant improvements in exercise capacity, symptoms of dyspnoea and fatigue, quality of life and reductions in hospital readmission have been documented in Cochrane reviews of randomized controlled trials of pulmonary rehabilitation (1,2).
Recent guidelines (3,4) and statements (5) from major respiratory organisations have outlined the key elements of pulmonary rehabilitation which include supervised exercise training programs of at least 2-3 sessions per week for 6-8 weeks in duration. Guidelines recommend that the exercise session include endurance exercise training and resistance training (3,4).
Endurance training
Physiological studies have demonstrated that lower limb endurance training in people with COPD can decrease ventilatory demand at a given level of exercise due to changes at the muscle level that include increased muscle fibre capillarisation (6,7), mitochondrial density and muscle oxidative capacity (8-10). Such training adaptations improve the aerobic capacity of the muscle, delaying the onset of lactic acidosis and, as such, reduce the ventilatory requirements for exercise at equivalent pre-training work rates (11). This is reflected in the ability of people with COPD to perform equivalent work rates for longer after training as well as achieving higher peak work rates (11).
In addition to lower limb endurance training, endurance training of the upper limb using either supported arm exercise (arm cranking) or unsupported arm exercise (free weights) has been shown to reduce ventilatory demand and improve arm exercise capacity (12-14). Such improvements are task specific (15), therefore exercise mimicking daily activities may be of greater functional relevance for people with COPD (16,17).
Resistance training
Reductions in skeletal muscle strength are evident in people with COPD (18,19) and may affect the ability to perform functional activities. Resistance training improves strength in people with COPD (20-22). Importantly, gains in strength may improve the performance of functional tasks, such as stair climbing and standing from a chair (21). Resistance training may also improve endurance capacity. Significant improvements in cycle endurance capacity were demonstrated in studies comparing strength training with no intervention (20,22). However, the gains in endurance capacity from resistance training were small compared to those that could be elicited from endurance training (20,22).
Besides improved exercise capacity, pulmonary rehabilitation programs have been shown to significantly improve health-related quality of life (1). It should be noted that while exercise training is considered the key component to achieve changes in exercise capacity and quality of life, exercise training often occurs as part of a comprehensive pulmonary rehabilitation program which includes education, anxiety and dyspnoea management, smoking cessation support, and nutritional advice. These additional components of pulmonary rehabilitation may enhance the outcomes of an exercise training regimen. However, a recent large randomised controlled trial of the addition of education to an exercise training program compared to exercise training alone showed no between group differences in exercise capacity, quality of life, physician visits, medication use or hospital admissions (23).
Access to pulmonary rehabilitation
Despite the high level evidence of the effectiveness of pulmonary rehabilitation, access to pulmonary rehabilitation programs worldwide is low. While reliable data on access to pulmonary rehabilitation programs for people with symptomatic COPD is not easily available, a number of studies have estimated that only 2-5% of people with COPD who could benefit from pulmonary rehabilitation have access to programs (24-26).
Most studies of the effectiveness of pulmonary rehabilitation have been performed in large metropolitan centres with well-equipped gymnasiums. There is a need to evaluate whether low cost programs with minimal equipment can achieve similar benefits. If programs providing pulmonary rehabilitation with minimal equipment are shown to be effective, the availability of pulmonary rehabilitation may be improved.
Exercise programs using minimal equipment
There have been a number of randomised controlled trials of endurance exercise training compared to standard care (no exercise training) in people with COPD in which the training mode required only minimal equipment [for example walking exercise (27-33), sit-to-stand (34), stepping (28,34)]. These trials were identified either from the most recent Cochrane review of pulmonary rehabilitation (1) or new trials published (in English) since that review based on a systematic search of Medline and Physiotherapy Evidence Database (PEDro) databases to identify randomised controlled trials of pulmonary rehabilitation where low resources were used. Table 1 provides a description of the interventions in these trials.
Full table
The methodological quality of the trials was determined by the PEDro score (35). The PEDro score is a valid (36) and reliable (37) measure of the methodological quality of a clinical trial. The PEDro score out of ten is based on a criterion that considers the internal validity of the trial and whether the trial has adequate statistical data to make it interpretable (35). If there was sufficient data for common outcome measures from a number of trials, a meta-analysis was performed. For any meta-analysis, the weighted mean differences (WMD) were determined from the difference between the pre- and post-intervention changes in the intervention and control groups. If change scores had not been presented in the study they were determined by subtracting the post-intervention means from the baseline means. If the standard deviation of change scores was missing, the standard deviations (SD) of the baseline and post-intervention means were pooled according to the equation: SD of mean change scores =√((SD2post + SD2baseline)/2) (38).
Exercise capacity
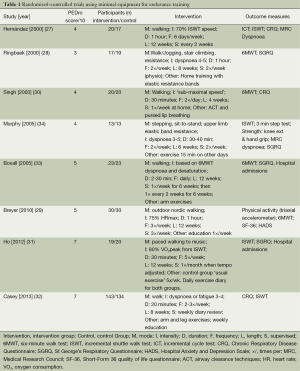
The studies of supervised lower limb endurance training in which minimal equipment has been used have mostly prescribed walking training (27-33). Four of these studies used the six-minute walk test (6MWT) to evaluate whether these training modes improved functional exercise capacity (28-30,33).
For the 6MWT, a meta-analysis included 90 participants in the exercise training group and 92 in the control group and showed a mean difference of 40 (95% CI: 13 to 67) metres in favour of the exercise group (Figure 1A). This difference in 6MWT distance was greater than the minimal important difference (MID) for the 6MWT which has been reported as 25 (95% CI: 20 to 61) metres (39). These combined studies suggest that walking training is adequate to improve functional exercise capacity.
Four studies used the incremental shuttle walk test as a measure of change in peak exercise capacity (27,31,32,34). For the ISWT, a meta-analysis included 195 participants in the exercise training group and 194 in the control group and showed no significant difference between the exercise group and the control group [mean difference 21 (95% CI: –9 to 51) metres] (Figure 1B). The ISWT is a measure of peak exercise capacity and an improvement requires the ability to walk faster. It may be that people with COPD who trained with minimal equipment can walk for longer after exercise training but may not be able to increase walking speed. None of these studies used the endurance shuttle walk test (40) as an outcome measure, which has been shown to be more sensitive to change following pulmonary rehabilitation (41).
The physiological changes induced by walking training have not been well studied. One study of predominately high intensity walking training in 25 participants with COPD demonstrated a significant reduction in lactate and ventilation at isotime on an incremental cycle test after walking training (42). These data provide some evidence of physiological responses in skeletal muscle due to walking training. In contrast, 20 participants in an eight-week, self-monitored, 5 days/week, home walking training program showed no reduction in lactate or ventilation on a constant work rate treadmill test after training (43). Randomised controlled trials with larger sample sizes are required to demonstrate whether physiological changes during a walking test are elicited after walking training.
Quality of life
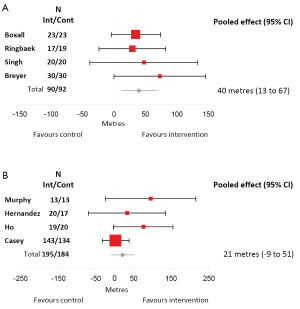
Quality of life is an important outcome measure for pulmonary rehabilitation. The most commonly used health-related quality of life questionnaires in the included studies were the Chronic Respiratory Disease Questionnaire (CRQ) (44) and the St George’s Respiratory Questionnaire (SGRQ) (45). Three studies reported the dyspnoea and fatigue domains of CRQ (27,30,32). A meta-analysis of these studies which included 183 participants in the exercise group and 171 in the control group showed a significant difference in dyspnoea of 0.48 (95% CI: 0.2 to 0.7) points (Figure 2A) and fatigue of 0.42 (0.2 to 0.7) points (Figure 2B) in favour of the exercise group. While these improvements were statistically significant, the MID for dyspnoea and fatigue is 0.5 points (46). Therefore, it could be considered that exercise training with minimal equipment resulted in a borderline clinically important improvement in the dyspnoea domain of the CRQ, however did not quite reach the MID for the fatigue domain. These mean differences are also less than those reported for studies that included training with exercise equipment (1).
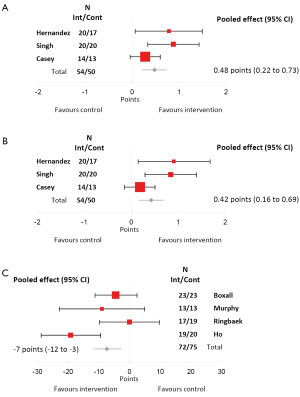
The SGRQ gives a total score for quality of life and four of the included studies used this as an outcome measure (28,31,33,34). A meta-analysis which included 72 participants in the exercise group and 75 in the control group showed a mean difference in quality of life of –7 (95% CI: –12 to –3) points in favour of the exercise group (Figure 2C). A lower score in the SGRQ indicates better quality of life and the MID for SGRQ total score is –4 points (47), indicating that exercise training with minimal equipment is adequate to achieve clinically relevant improvements in health-related quality of life.
Prescribing walking training
A number of the studies in Table 1 prescribed walking intensity based on symptoms of dyspnoea (28,32,33). However, intensity of walking training can be prescribed from the initial field walking tests of either the 6MWT or the ISWT. Walking at 80% of the average 6MWT speed has been shown to elicit a mean (± SD) oxygen uptake (VO2) of 77% (±13) of VO2peak (48), whereas walking at 70% peak ISWT speed has been has been shown to elicit an oxygen uptake (VO2) of 76% (±11) of VO2peak (49). Exercise training above 50% VO2peak is recommended, as exercise above this intensity is usually sufficient to achieve physiological training effects (50), with higher intensities possibly achieving greater training responses (11,51).
Resistance training
Three randomised controlled trials were identified that used minimal equipment for resistance training (52-54). One study compared a 12-week program of once a week supervised and twice a week home-based resistance training exercises such as sit-to-stand, seated row, lunges, simulated lifting, chest press using elasticised resistance bands, compared to no training (53). The trial quality was a PEDro score of 7. Results showed a small mean difference in knee extensor strength in favour of the exercise group with no differences in other outcomes such as 6MWT or health-related quality of life. More recently a high quality randomised controlled trial (PEDro score 8) of supervised elastic band resistance training plus patient education three times per week for eight weeks compared to patient education alone reported significant mean differences in 6MWT, unsupported arm exercise, and muscle strength in favour of the exercise group (52). Interestingly, a study that compared elastic resistance band training to conventional equipment-based resistance training demonstrated that both groups improved strength with no differences between groups (PEDro score 6) (54). Although these studies only equate to limited evidence, the findings suggest that supervised training with resistance bands may be an appropriate substitute for equipment-based resistance training in people with COPD.
Other less conventional training modes using minimal equipment
Tai Chi
Tai Chi is an ancient Chinese martial art which incorporates elements of strengthening, balance, postural alignment and concentration. It represents a mode of training which does not require a specific training venue, can be performed without exercise equipment, and promotes aerobic, strength and balance training simultaneously. While there has been a recent systematic review of Tai Chi in COPD the included studies were of low methodological quality (55). Recently, a randomised controlled trial of Tai Chi in COPD with a high methodological quality (Pedro score 8) (56) demonstrated that Tai Chi training resulted in a significant difference in endurance shuttle walk test (ESWT) time and SGRQ Total score in favour of the Tai Chi group compared to the control group (no exercise training) [ESWT mean difference 384 (95% CI: 186 to 510) seconds; SGRQ Total score –11 (95% CI: –18 to –4) points]. These between group differences exceeded the minimum clinically important differences for these outcomes. Balance and quadriceps strength were also significantly increased in the Tai Chi group compared to the control group. Importantly, Tai Chi elicited a VO2 of approximately 63% VO2peak which would be adequate to achieve physiological training effects (54). This study provides some support for Tai Chi as a mode of training to improve exercise capacity and health-related quality of life in COPD.
Further research is needed with larger randomised controlled trials comparing pulmonary rehabilitation programs using minimal equipment with programs using gymnasium equipment. Such studies will help to determine the effects of minimal equipment programs benchmarked against the standard programs. In addition, the effects of minimal equipment on the longer term maintenance of benefits should be evaluated. It is possible that programs using minimal equipment may transfer to the home environment more easily and promote continued exercise and hence maintenance of benefits.
Conclusions
The demand for pulmonary rehabilitation and the lack of available programs requires focus on alternatives to conventional equipment-based exercise training that can be more widely offered. There is growing evidence that exercise training using minimal equipment is effective in improving outcomes of functional exercise capacity and health-related quality of life in people with COPD.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Lacasse Y, Goldstein R, Lasserson TJ, et al. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2006;CD003793. [PubMed]
- Puhan MA, Gimeno-Santos E, Scharplatz M, et al. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;CD005305. [PubMed]
- Bolton CE, Bevan-Smith EF, Blakey JD, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68 Suppl 2:ii1-30. [PubMed]
- Ries AL, Bauldoff GS, Carlin BW, et al. Pulmonary Rehabilitation: Joint ACCP/AACVPR Evidence-Based Clinical Practice Guidelines. Chest 2007;131:4S-42S. [PubMed]
- Spruit MA, Singh SJ, Garvey C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. Am J Respir Crit Care Med 2013;188:e13-64. [PubMed]
- Eliason G, Abdel-Halim SM, Piehl-Aulin K, et al. Alterations in the muscle-to-capillary interface in patients with different degrees of chronic obstructive pulmonary disease. Respir Res 2010;11:97. [PubMed]
- Whittom F, Jobin J, Simard PM, et al. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 1998;30:1467-74. [PubMed]
- Maltais F, LeBlanc P, Simard C, et al. Skeletal muscle adaptation to endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1996;154:442-7. [PubMed]
- McKeough ZJ, Alison JA, Bye PT, et al. Exercise capacity and quadriceps muscle metabolism following training in subjects with COPD. Respir Med 2006;100:1817-25. [PubMed]
- Sala E, Roca J, Marrades RM, et al. Effects of endurance training on skeletal muscle bioenergetics in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1999;159:1726-34. [PubMed]
- Casaburi R, Patessio A, Ioli F, et al. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. Am Rev Respir Dis 1991;143:9-18. [PubMed]
- Couser JI Jr, Martinez FJ, Celli BR. Pulmonary rehabilitation that includes arm exercise reduces metabolic and ventilatory requirements for simple arm elevation. Chest 1993;103:37-41. [PubMed]
- Epstein SK, Celli BR, Martinez FJ, et al. Arm training reduces the VO2 and VE cost of unsupported arm exercise and elevation in chronic obstructive pulmonary disease. J Cardiopulm Rehabil 1997;17:171-7. [PubMed]
- McKeough ZJ, Bye PT, Alison JA. Arm exercise training in chronic obstructive pulmonary disease: a randomised controlled trial. Chron Respir Dis 2012;9:153-62. [PubMed]
- Martinez FJ, Vogel PD, Dupont DN, et al. Supported arm exercise vs unsupported arm exercise in the rehabilitation of patients with severe chronic airflow obstruction. Chest 1993;103:1397-402. [PubMed]
- Ennis S, Alison J, McKeough Z. The effects of arm endurance and strength training on arm exercise capacity in people with chronic obstructive pulmonary disease. Physical Therapy Reviews 2009;14:226-39.
- Janaudis-Ferreira T, Hill K, Goldstein R, et al. Arm exercise training in patients with chronic obstructive pulmonary disease: a systematic review. J Cardiopulm Rehabil Prev 2009;29:277-83. [PubMed]
- Gosselink R, Troosters T, Decramer M. Peripheral muscle weakness contributes to exercise limitation in COPD. Am J Respir Crit Care Med 1996;153:976-80. [PubMed]
- Hamilton AL, Killian KJ, Summers E, et al. Muscle strength, symptom intensity, and exercise capacity in patients with cardiorespiratory disorders. Am J Respir Crit Care Med 1995;152:2021-31. [PubMed]
- Ortega F, Toral J, Cejudo P, et al. Comparison of effects of strength and endurance training in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2002;166:669-74. [PubMed]
- O’Shea SD, Taylor NF, Paratz JD. Progressive resistance exercise improves muscle strength and may improve elements of performance of daily activities for people with COPD: a systematic review. Chest 2009;136:1269-83. [PubMed]
- Spruit MA, Gosselink R, Troosters T, et al. Resistance versus endurance training in patients with COPD and peripheral muscle weakness. Eur Respir J 2002;19:1072-8. [PubMed]
- Blackstock FC, Webster KE, McDonald CF, et al. Comparable improvements achieved in chronic obstructive pulmonary disease through pulmonary rehabilitation with and without a structured educational intervention: A randomized controlled trial. Respirology 2014;19:193-202. [PubMed]
- Brooks D, Sottana R, Bell B, et al. Characterization of pulmonary rehabilitation programs in Canada in 2005. Can Respir J 2007;14:87-92. [PubMed]
- Yohannes AM, Connolly MJ. Pulmonary rehabilitation programmes in the UK: a national representative survey. Clin Rehabil 2004;18:444-9. [PubMed]
- Wadell K, Janaudis Ferreira T, Arne M, et al. Hospital-based pulmonary rehabilitation in patients with COPD in Sweden--a national survey. Respir Med 2013;107:1195-200. [PubMed]
- Hernández MT, Rubio TM, Ruiz FO, et al. Results of a home-based training program for patients with COPD. Chest 2000;118:106-14. [PubMed]
- Ringbaek TJ, Broendum E, Hemmingsen L, et al. Rehabilitation of patients with chronic obstructive pulmonary disease. Exercise twice a week is not sufficient! Respir Med 2000;94:150-4. [PubMed]
- Breyer MK, Breyer-Kohansal R, Funk GC, et al. Nordic walking improves daily physical activities in COPD: a randomised controlled trial. Respir Res 2010;11:112. [PubMed]
- Singh V, Khandelwal DC, Khandelwal R, et al. Pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Indian J Chest Dis Allied Sci 2003;45:13-7. [PubMed]
- Ho CF, Maa SH, Shyu YI, et al. Effectiveness of paced walking to music at home for patients with COPD. COPD 2012;9:447-57. [PubMed]
- Casey D, Murphy K, Devane D, et al. The effectiveness of a structured education pulmonary rehabilitation programme for improving the health status of people with moderate and severe chronic obstructive pulmonary disease in primary care: the PRINCE cluster randomised trial. Thorax 2013;68:922-8. [PubMed]
- Boxall AM, Barclay L, Sayers A, et al. Managing chronic obstructive pulmonary disease in the community. A randomized controlled trial of home-based pulmonary rehabilitation for elderly housebound patients. J Cardiopulm Rehabil 2005;25:378-85. [PubMed]
- Murphy N, Bell C, Costello RW. Extending a home from hospital care programme for COPD exacerbations to include pulmonary rehabilitation. Respir Med 2005;99:1297-302. [PubMed]
- PEDro scale. 1999 [cited 2014, 5 June]; Available online: http://www.pedro.org.au/english/faq/#question_five
- de Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother 2009;55:129-33. [PubMed]
- Maher CG, Sherrington C, Herbert RD, et al. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther 2003;83:713-21. [PubMed]
- Dunst CJ, Hamby DW, Trivette CM. Guidelines for calculating effect sizes for practice-based research syntheses. Centrescope 2004;3:1-10.
- Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010;91:221-5. [PubMed]
- Revill SM, Morgan MD, Singh SJ, et al. The endurance shuttle walk: a new field test for the assessment of endurance capacity in chronic obstructive pulmonary disease. Thorax 1999;54:213-22. [PubMed]
- Eaton T, Young P, Nicol K, et al. The endurance shuttle walking test: a responsive measure in pulmonary rehabilitation for COPD patients. Chron Respir Dis 2006;3:3-9. [PubMed]
- Calvert LD, Singh SJ, Morgan MD, et al. Exercise induced skeletal muscle metabolic stress is reduced after pulmonary rehabilitation in COPD. Respir Med 2011;105:363-70. [PubMed]
- Puente-Maestu L, Sánz ML, Sánz P, et al. Comparison of effects of supervised versus self-monitored training programmes in patients with chronic obstructive pulmonary disease. Eur Respir J 2000;15:517-25. [PubMed]
- Guyatt GH, Berman LB, Townsend M, et al. A measure of quality of life for clinical trials in chronic lung disease. Thorax 1987;42:773-8. [PubMed]
- Jones PW, Quirk FH, Baveystock CM. The St George’s Respiratory Questionnaire. Respir Med 1991;85 Suppl B:25-31; discussion 33-7.
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Control Clin Trials 1989;10:407-15. [PubMed]
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. Eur Respir J 2002;19:398-404. [PubMed]
- Zainuldin R, Mackey MG, Alison JA. Prescription of Walking Exercise Intensity From the 6-Minute Walk Test in People With Chronic Obstructive Pulmonary Disease. J Cardiopulm Rehabil Prev 2014. [Epub ahead of print]. [PubMed]
- Zainuldin R, Mackey MG, Alison JA. Prescription of walking exercise intensity from the incremental shuttle walk test in people with chronic obstructive pulmonary disease. Am J Phys Med Rehabil 2012;91:592-600. [PubMed]
- Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc 2011;43:1334-59. [PubMed]
- Zainuldin R, Mackey MG, Alison JA. Optimal intensity and type of leg exercise training for people with chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2011;CD008008. [PubMed]
- Nyberg A, Lindström B, Rickenlund A, et al. Low-load/high-repetition elastic band resistance training in patients with COPD: a randomized, controlled, multicenter trial. Clin Respir J 2014. [Epub ahead of print].
- O’Shea SD, Taylor NF, Paratz JD. A predominantly home-based progressive resistance exercise program increases knee extensor strength in the short-term in people with chronic obstructive pulmonary disease: a randomised controlled trial. Aust J Physiother 2007;53:229-37. [PubMed]
- Ramos EM, de Toledo-Arruda AC, Fosco LC, et al. The effects of elastic tubing-based resistance training compared with conventional resistance training in patients with moderate chronic obstructive pulmonary disease: a randomized clinical trial. Clin Rehabil 2014;28:1096-106. [PubMed]
- Ding M, Zhang W, Li K, et al. Effectiveness of t’ai chi and qigong on chronic obstructive pulmonary disease: a systematic review and meta-analysis. J Altern Complement Med 2014;20:79-86. [PubMed]
- Leung RW, McKeough ZJ, Peters MJ, et al. Short-form Sun-style t’ai chi as an exercise training modality in people with COPD. Eur Respir J 2013;41:1051-7. [PubMed]


