
The use of magnetic resonance imaging in differential diagnosis of allergic fungal sinusitis and eosinophilic mucin rhinosinusitis
Introduction
Millar and colleagues (1) first described “allergic aspergillosis of the paranasal sinuses”. Katzenstein and colleagues (2) subsequently proposed the term “allergic aspergillus sinusitis” and demonstrated that the pathological features of this condition comprised the presence of clusters of necrotic eosinophils, Charcot-Leyden crystals, and septate fungal hyphae in the sinus mucus of patients. Although Aspergillus species were initially suspected as the causative agent, Robson and colleagues (3) demonstrated that other, non-Aspergillus fungi were also involved in the pathology of the condition and thus introduced the term “allergic fungal sinusitis (AFS)”.
The recognition and incidence of AFS have increased markedly over the last 2 decades (4), and it has been postulated that immunoglobulin E (IgE)-mediated and type III hypersensitivity to fungi in an atopic host may be the major pathogenic mechanism underlying AFS (5). The main diagnostic criteria for AFS are (I) IgE-mediated hypersensitivity, (II) polyposis, (III) typical computed tomography (CT) scan findings (showing the presence of intrasinus hyperattenuating material), (IV) eosinophilic mucin, and (V) positive fungal culture or staining (6). Depending on the extent of fungal colonization, AFS may be unilateral or bilateral (7). However, the CT finding of AFS with bilateral nasal polyps is often confused with eosinophilic mucus rhinosinusitis (EMRS), because both these conditions show intrasinus hyperattenuating material on CT scans due to “eosinophilic mucus” (8). To date, the above criteria have not included the use of magnetic resonance imaging (MRI) in the diagnosis of AFS.
Eosinophilic chronic rhinosinusitis (ECRS) is a major subset of chronic rhinosinusitis (CRS), and the main manifestation is thick, tenacious, eosinophilic mucus in the sinuses (5,8). Clinically, EMRS is a systemic disease associated with the upper and lower respiratory tract (9). EMRS patients usually present with bilateral nasal polyps and sticky mucus secretion in the affected sinuses and are susceptible to asthma, dysosmia, and nasal congestion, which impair their quality of life (10). Similar to AFS patients, CT scans of most EMRS patients show intrasinus hyperattenuating material due to “eosinophilic mucus” (8). Furthermore, EMRS is refractory to medical and surgical interventions and recurrence is common (5,11).
EMRS was first described as a subtype of sinusitis that resembles AFS histologically and clinically (12). However, AFS and EMRS represent pathophysiological variations of a clinical phenotype, although some recent studies have reported that they are distinct clinical entities (8,9). While it is comparatively easy to differentiate unilaterally involved AFS from EMRS by routine clinical examination, it is not easy to differentiate bilateral AFS from EMRS in the clinic, because many distinguishing clinical features of AFS, such as polyposis, typical CT scan findings, and eosinophilic mucin, are similar to the clinical features of EMRS (2). However, an inaccurate diagnosis of these different conditions can lead to inefficient therapy, prior to and following surgery.
We hypothesized that MRI could improve the diagnostic accuracy in AFS and EMRS. To date, few studies (8,9,12,13) have reported on the differential diagnosis of AFS and EMRS in detail. Thus, in this study, we investigated the use of MRI in the differential diagnosis of AFS and EMRS.
Methods
Study design and patient population
A prospective study of consecutive patients undergoing endoscopic sinus surgery (ESS) at Beijing Tongren Hospital, from February 2013 to September 2016, was conducted. During surgery, we took photographs of the secretion of AFS and EMRS patients. Patients with rhinosinusitis underwent CT scanning before surgery (Philips Health Care, Best, The Netherlands) and only patients who had bilateral nasal polyps and eosinophilic mucin in the sinuses, in addition to high attenuation within the opacified sinuses on CT scans, were recruited consecutively. Overall, 93 eligible patients with sinusitis were enrolled in the study and were evaluated further for a diagnosis of AFS or EMRS. The diagnosis of AFS was based on the criteria set out by Bent and Kuhn (6) as follows: (I) IgE-mediated hypersensitivity, (II) polyposis, (III) typical CT scan findings (intrasinus hyperattenuating material), (IV) eosinophilic mucin, and (V) positive fungal culture or staining. The diagnosis of EMRS was based on a histological criterion of a tissue eosinophil count ≥27%, as suggested by Lou and colleagues (14), and a negative culture for fungus in the nasal secretions and mucosa.
The study was conducted in full accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of Beijing Tongren Hospital and all patients provided written informed consent prior to collection of data.
Clinical and laboratory examinations
Prior to surgery, all patients underwent MRI on a 1.5-T unit (Signa Twin Speed Excite, GE Healthcare, Chicago, IL, USA) or a 3.0-T unit (SignaHDx, GE Healthcare) with an 8-channel head coil. Precontrast axial and coronal T1-weighted spin-echo images and T2-weighted fast spin-echo images were obtained, followed by contrast-enhanced axial, coronal, and sagittal T1-weighted spin-echo images, after intravenous injection of 0.1 mmol/kg of gadopentetate dimeglumine (Magnevist, Schering, Berlin, Germany). Contrast-enhanced T1-weighted imaging with frequency-selective, fat saturation was performed in the axial plane. All images were analyzed by 2 independent radiologists with more than 10 years of experience in head and neck imaging. Both individuals were blinded to the medical histories and diagnoses of the patients.
A complete blood cell count with differential cell count was performed within 1 week before surgery, when the patient was well and without any apparent infection. The percentage of eosinophils and absolute blood eosinophil counts were determined as differential factors. Allergy to fungi was confirmed based on the presence of specific IgE (sIgE), detected by Immuno-Cap Phadiatop (Pharmacia, Uppsala, Sweden) (cut-off ≥0.35 kU/mL).
Fungal culture
Samples of nasal secretions were cultured for fungi in liquid Sabouraud medium, agar slant Sabouraud medium, and Sabouraud dextrose agar medium. The media were incubated at 26 °C for 3 weeks for fungal growth and the established fungal colonies were identified by morphological phenotype.
Pathological examinations
Ethmoid sinus mucosa samples were obtained from each patient during ESS and processed for histological evaluation using standard techniques. Four-micrometer-thick sections were stained with hematoxylin and eosin (H&E) stain and assessed by light microscopy for the presence of eosinophils, neutrophils, plasma cells, lymphocytes, and edema.
Statistical analysis
All statistical analyses were performed with GraphPad Prism software (GraphPad Software, Inc. La Jolla, CA) and SPSS for Windows version 21.0 (IBM Corp., Armonk, NY, USA). The two-sample t-test and chi-square test were used for 2-group comparison of age, sex ratio, the onset of asthma, history of allergy, and presence of MRI signal loss. A one-way analysis of variance was followed by a Mann-Whitney test for a 2-group comparison of blood eosinophil counts. Differences were considered to be significant for P<0.05. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each parameter, and receiver operating characteristic (ROC) curves were used to assess the predictive value of clinical factors. The diagnostic ability of each clinical factor was calculated based on the area under the ROC curve (AUC); with an AUC value close to 1 indicating high predictability. An AUC value greater than 0.9 is considered to represent high accuracy, and AUC values of 0.7–0.9 and 0.5–0.7 represent moderate and low accuracies, respectively (15). Five parameters; including the onset of asthma, history of allergy, presence of MRI signal loss, blood eosinophil percentage, and blood eosinophil absolute count, which might be useful for predicting the onset of AFS, were assessed using logistic regression analysis. Furthermore, ROC curves were compared for 2 logistic models, of which 1 model included all the clinical parameters measured, except MRI signal loss (model 1), and the other model included all 5 clinical parameters measured (model 2).
Results
The demographic and clinical characteristics of patients are shown in Table 1. The AFS and EMRS patient groups did not differ significantly with respect to either percentage of blood eosinophils or absolute blood eosinophil counts. However, the age distribution, sex ratio, and presence of asthma were significantly higher in the EMRS group, whereas allergy to fungi, and T2-weighted MRI signal loss were significantly higher in the AFS group.

Full table
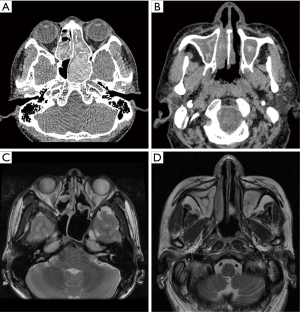
There was no significant difference on the degree of inter-observer agreement between the 2 radiologists. Both AFS and EMRS patients showed the presence of hyperattenuating material within the affected sinuses on CT scans, which was more prominent in soft tissue windows (Figure 1A,B). In contrast, MRI T2-weighted sequences of AFS showed peripheral hyperintense signals concomitant with markedly hypointense signals or signal loss in the center, but the T2-weighted sequences of EMRS showed an extended hyperintense signal in affected sinuses, without hypointense signals or signal loss (Figure 1C,D).
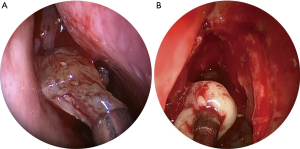
In AFS patients, the mucin was thick, highly viscous, and greenish-brown in color (Figure 2A). EMRS patients produced a white, viscous, mucinous secretion in sinus cavities, without the presence of any fungal components (Figure 2B).
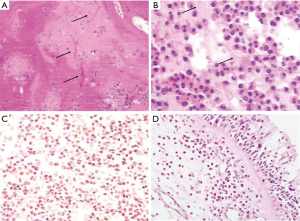
Microscopically, the mucin of AFS patients was chondroid in appearance, with abundant sheets of eosinophils, and sometimes contained Charcot-Leyden crystals or hyphae (Figure 3A,B). Secretions obtained from EMRS patients showed large numbers of eosinophils, but no hyphae (Figure 3C). Similarly, tissue samples obtained from EMRS patients were also infiltrated by large numbers of eosinophils, however, the numbers of cells in the field appeared to be fewer than that observed in samples of AFS patients, because of the presence of edema (Figure 3D).
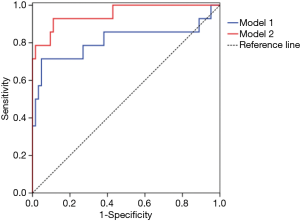
The AUC of sIgE, percentage of blood eosinophils, and absolute blood eosinophil counts are shown in Table 2. The AUC of model 1 was 0.845, which indicated that the model had a moderate predictive accuracy for AFS. In contrast, the AUC of model 2 was 0.951, indicating a higher predictive accuracy for AFS (Table 2, Figure 4).

Full table
Discussion
Eosinophils are considered to play a major role both in the pathogenesis of AFS and EMRS (16,17). The presenting clinical complaints of these 2 conditions are also usually nonspecific and consist primarily of symptoms of CRS, including nasal obstruction, nasal discharge, and sneezing (5), thus often making differential diagnosis of these 2 diseases difficult, especially in patients with bilateral disease. However, studies have recently reported that AFS and EMRS are 2 distinct conditions (8,9) with AFS being an IgE-mediated fungal allergic disease (8), and EMRS being characterized by Th2-polarized inflammation and marked expression of eotaxins, as well as tissue remodeling and eosinophilia, dependent upon IL-17A expression (13).
Our study indicated that patients with AFS generally tended to be significantly younger (mean age =29.2 years) than patients with EMRS (mean age =42 years, P<0.001). More female than male subjects were affected by either of these conditions, and this female predilection was greater in the AFS than in the EMRS (female to male ratio =1.72 in the AFS group vs. 1.42 in the EMRS group). These findings were in accordance with the findings of Pant and colleagues (8) and Ferguson (9). The study by Ferguson (9) is particularly interesting because it compared cases of AFS (n=418) to those with EMRS (n=40) reported in the literature, as well as cases of AFS (n=13) and EMRS (n=29) accrued in the author’s own study. Ferguson reported that the mean age of patients with AFS (30.7 years) was significantly lower than that of patients with EMRS (48.0 y; P<0.001). Similarly, the male: female ratio was also lower for AFS patients (1.03:1) than for EMRS patients (1.26:1).
The finding in the present study that the number of patients with incidental asthma was significantly (2-fold) higher among EMRS patients (61.9%) than among AFS patients (30.0%) was also in accordance with the findings of other studies (9,12). While the study by Ferguson (9) demonstrated the presence of asthma in 93% EMRS patients, as compared with 41% of AFS patients, another study by Hutcheson and colleagues (12) demonstrated the presence of asthma in 73% of EMRS patients as compared with 37% of AFS patients.
Although AFS and EMRS are both associated with eosinophilia, a systemic dysregulation of total airway eosinophilia, rather than fungal antigen appears to contribute to the occurrence of EMRS (9). Thus, while the lower airway is usually affected in EMRS patients, this is not always the case in AFS patients. However, our finding of the presence of significantly higher levels of fungal sIgE in AFS patients than in EMRS patients was in accordance with the findings of others (16,18). Indeed, a review of studies investigating patients with AFS has suggested that the presence of a fungal allergen was a predominant characteristic feature in this group of patients (19). In the present study, all the patients with AFS demonstrated the presence of sIgE to fungi, whereas only 10 patients with EMRS had this characteristic. Despite the positivity for sIgE for fungal allergens in these EMRS patients, we had confirmed that fungi had not colonized or pathologically affected the sinuses; i.e., the fungal allergen did not induce AFS in these 10 EMRS patients.
CT scans showed that all the patients enrolled in the present study were affected bilaterally. All patients in both groups had eosinophilic mucin within the affected sinuses, and we were able to observe intra-sinus hyperattenuating material on CT scans (20), which was more obvious in soft tissue windows. Although CT can also be used to calculate other features, such as bone erosion and remodeling, sinus expansion, and nasal polyp formation, and has been employed as an important tool for the diagnosis of sinusitis, it is limited in its use for assessment of sinusitis with complications (21), and therefore the use of an additional diagnostic tool, such as MRI, may be required.
MRIs were available for all 93 patients in the present study. In AFS patients, there was no specific imaging feature on T1-weighted images. This may be due to the difference in the protein concentration of the mucin in the sinuses, which was greenish-black, whitish-tan or brown, with the consistency of cottage cheese in endoscopy (5), and the proteinaceous secretion showed characteristic changes from a loose mucous collection to a stone-like mucous plug (22). In contrast, T2-weighted sequences showed peripheral hyperintense signals, concomitant with the markedly hypointense signal or signal attenuation within the center of the sinuses, likely due to the obstructive inflammation and the increased concentration of paramagnetic heavy metals (fungal metabolites), high protein concentration, and low free-water content of the allergic mucin (20). This finding was in accordance with those of Manning and colleagues (23). Furthermore, the signal loss within the center of the sinuses on T2-weighted sequences corresponded with the central hyperdensity (high-attenuation areas) observed on CT scans, which is a characteristic of AFS (20) (Figure 1C). In the EMRS group, mucosa edema and mucus accumulation were the main pathological changes, and thus, on T2-weighted images, extended hyperintense signals were observed in the affected sinuses, which represent the inflamed mucosa (19) (Figure 1D). Although there may be some differences in signals due to differences in the concentration of secretion in the sinus, this is unlikely to interfere with the diagnosis of EMRS.
In our experience, MRI assisted in the differential diagnosis of these 2 conditions. Firstly, fungus is an opportunistic pathogen; AFS onset occurs with fungal colonization. If AFS patients are not infected with fungi after surgery, the condition may not recur. However, EMRS is a systemic dysregulation involving total airway eosinophilia, rather than a response to fungal antigen (9). According to Lou et al., if the tissue eosinophil count was equal to or greater than 27%, the recurrence rate was higher than 90% (14). Thus, we recommend that patients who have intra-sinus hyperattenuating material on CT scans (20), negative fungal smears, and negative fungal cultures should undergo an MRI examination, to predict prognosis and recurrence of the disease, and to ensure use of condition-appropriate treatment. Secondly, the positive rate of fungal culture is not 100% (18,19), while fungi are also present in healthy individuals and in AFS patients after surgical treatment (19). However, MRI had a high diagnosis rate (100%) for AFS in the present study. Thirdly, the relatively high cost of MRI examination is a limitation to its clinical application, but if MRI is only used for ambiguous cases, medical expenses can be reduced.
A recent review of studies investigating the current diagnosis, pathogenesis, and treatment of AFS, by Glass and Amedee (24), has indicated that examination of the unique allergic fungal mucin itself, and not the surrounding mucosa, is the most reliable indicator of disease. In AFS patients, the mucin has been shown to be thick, highly viscous, and greenish-brown, greenish-black, or yellow in color (Figure 2A), and has been described to have a peanut butter-like consistency in the nasal or sinus cavities, due to fungal metabolism (22,25). The mucin also contains Charcot-Leyden crystals, which are a by-product of necrotic eosinophils (26), and fungal hyphae (18). Dematiaceous fungi are thought to be the most common etiological agents involved in AFS. These include Bipolaris and Alternaria species, which are the fungi most often cultured from patients, followed by Aspergillus (27,28). In the present study, 19 (63%) AFS patients demonstrated a positive result for fungal culture; of which Aspergillus was cultured from 14 patients, Alternaria tenuis from 2 patients, and other species from 3 patients. In contrast, most EMRS patients produced a white, viscous, mucinous secretion in nasal or sinus cavities, as demonstrated in other studies (29), which lacked any fungal components (Figure 2B).
Microscopically, the mucin in AFS patients has been shown to take on a chondroid appearance with abundant sheets of eosinophilic cells, and frequently contains Charcot-Leyden crystals, as shown by H&E staining, and sometimes hyphae (24) (Figure 3A,B). In our study, the presence of active eosinophil degranulation was commonly seen in AFS patients, likely as a consequence of persistent hypersensitivity in these patients. In the mucosa, many other types of cells, such as mast cells, lymphocytes, and plasma cells, have been observed in addition to eosinophils (24). In addition, secretory gland hypertrophy is present in AFS tissue. Massive eosinophilic migration out of the tissue is characteristic in AFS patients and may lead to “cluster formation” due to activation of eosinophil degranulation (25). However, although large numbers of eosinophils were seen in the secretions of our EMRS patients, eosinophil degranulation was seldom seen by H&E staining. Similarly, the tissues of EMRS patients were also infiltrated by large numbers of eosinophils, although the numbers of these cells in the observation field were fewer than those noted in AFS, because of the presence of edema.
In the current study, the AUC of sIgE, as well as the percentage and absolute count of blood eosinophils, was very low and could not meet the clinical standards. We also employed 2 logistic regression models, 1 of which included all the clinical parameters measured, except MRI signal loss (model 1), and another which included all 5 clinical parameters measured (model 2). Assessment of the AUCs of the 2 models showed that, for model 1, the AUC was 0.845, indicating moderate accuracy, whereas for model 2, the AUC was 0.951, which showed higher accuracy for predicting AFS (Figure 4). Collectively, these findings suggest that MRI is likely to be an accurate and appropriate choice for differential diagnosis of AFS and EMRS in a clinical context.
Conclusions
Although AFS and EMRS can often be confused in the clinic, these 2 distinct conditions can be accurately diagnosed with the help of MRI.
Acknowledgments
Funding: This work was supported by National Key R&D Program of China (2016YFC20160905200); National Natural Science Foundation of China (81630023, 81400444, 81371104, 81470678, and 81420108009); Program for Changjiang Scholars and Innovative Research Team (IRT13082); Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support (ZYLX201310); Beijing Natural Science Foundation (Z141107002514122); Capital Health Development Foundation (2016-1-2052), and Beijing Talents Project (2016).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in full accordance with Declaration of Helsinki and was approved by the Medical Ethics Committee of Beijing TongRen Hospital (No. V1.0) and all patients provided written informed consent prior to collection of data.
References
- Millar JW, Johnston A, Lamb D. Allergic aspergillosis of the maxillary sinuses. Thorax 1981;36:710.
- Katzenstein AL, Sale SR, Greenberger PA. Allergic Aspergillus sinusitis: a newly recognized form of sinusitis. J Allergy Clin Immunol 1983;72:89-93. [Crossref] [PubMed]
- Robson JM, Hogan PG, Benn RA, et al. Allergic fungal sinusitis presenting as a paranasal sinus tumour. Aust N Z J Med 1989;19:351-3. [Crossref] [PubMed]
- McClay JE, Marple B, Kapadia L, et al. Clinical presentation of allergic fungal sinusitis in children. Laryngoscope 2002;112:565-9. [Crossref] [PubMed]
- Lee SH, Kim HJ, Lee JW, et al. Categorization and clinicopathological features of chronic rhinosinusitis with eosinophilic mucin in a korean population. Clin Exp Otorhinolaryngol 2015;8:39-45. [Crossref] [PubMed]
- Bent JP 3rd, Kuhn FA. Diagnosis of allergic fungal sinusitis. Otolaryngol Head Neck Surg 1994;111:580-8. [Crossref] [PubMed]
- Ferguson BJ. Categorization of eosinophilic chronic rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg 2004;12:237-42. [Crossref] [PubMed]
- Pant H, Kette FE, Smith WB, et al. Eosinophilic mucus chronic rhinosinusitis: clinical subgroups or a homogeneous pathogenic entity? Laryngoscope 2006;116:1241-7. [Crossref] [PubMed]
- Ferguson BJ. Eosinophilic mucin rhinosinusitis: a distinct clinicopathological entity. Laryngoscope 2000;110:799-813. [Crossref] [PubMed]
- Soler ZM, Sauer D, Mace J, et al. Impact of mucosal eosinophilia and nasal polyposis on quality-of-life outcomes after sinus surgery. Otolaryngol Head Neck Surg 2010;142:64-71. [Crossref] [PubMed]
- Shah SA, Ishinaga H, Takeuchi K. Pathogenesis of eosinophilic chronic rhinosinusitis. J Inflamm (Lond) 2016;13:11. [Crossref] [PubMed]
- Uri N, Ronen O, Marshak T, et al. Allergic fungal sinusitis and eosinophilic mucin rhinosinusitis: diagnostic criteria. J Laryngol Otol 2013;127:867-71. [Crossref] [PubMed]
- Ikeda K, Shiozawa A, Ono N, et al. Subclassification of chronic rhinosinusitis with nasal polyp based on eosinophil and neutrophil. Laryngoscope 2013;123:E1-9. [Crossref] [PubMed]
- Lou H, Meng Y, Piao Y, et al. Cellular phenotyping of chronic rhinosinusitis with nasal polyps. Rhinology 2016;54:150-9. [Crossref] [PubMed]
- Fischer JE, Bachmann LM, Jaeschke R. A readers’ guide to the interpretation of diagnostic test properties: clinical example of sepsis. Intensive Care Med 2003;29:1043-51. [Crossref] [PubMed]
- Hutcheson PS, Schubert MS, Slavin RG. Distinctions between allergic fungal rhinosinusitis and chronic rhinosinusitis. Am J Rhinol Allergy 2010;24:405-8. [Crossref] [PubMed]
- Takeno S, Hirakawa K, Ishino T. Pathological mechanisms and clinical features of eosinophilic chronic rhinosinusitis in the Japanese population. Allergol Int 2010;59:247-56. [Crossref] [PubMed]
- Chakrabarti A, Denning DW, Ferguson BJ, et al. Fungal rhinosinusitis: a categorization and definitional schema addressing current controversies. Laryngoscope 2009;119:1809-18. [Crossref] [PubMed]
- Marple BF. Allergic fungal rhinosinusitis: current theories and management strategies. Laryngoscope 2001;111:1006-19. [Crossref] [PubMed]
- Mossa-Basha M, Ilica AT, Maluf F, et al. The many faces of fungal disease of the paranasal sinuses: CT and MRI findings. Diagn Interv Radiol 2013;19:195-200. [PubMed]
- Younis RT, Anand VK, Davidson B. The role of computed tomography and magnetic resonance imaging in patients with sinusitis with complications. Laryngoscope 2002;112:224-9. [Crossref] [PubMed]
- Al-Dousary SH. Allergic fungal sinusitis: radiological and microbiological features of 59 cases. Ann Saudi Med 2008;28:17-21. [PubMed]
- Manning SC, Merkel M, Kriesel K, et al. Computed tomography and magnetic resonance diagnosis of allergic fungal sinusitis. Laryngoscope 1997;107:170-6. [Crossref] [PubMed]
- Glass D, Amedee RG. Allergic fungal rhinosinusitis: a review. Ochsner J 2011;11:271-5. [PubMed]
- Mukherji SK, Figueroa RE, Ginsberg LE, et al. Allergic fungal sinusitis: CT findings. Radiology 1998;207:417-22. [Crossref] [PubMed]
- Braun H, Buzina W, Freudenschuss K, et al. ‘Eosinophilic fungal rhinosinusitis’: a common disorder in Europe? Laryngoscope 2003;113:264-9. [Crossref] [PubMed]
- Schubert MS. Allergic fungal sinusitis: pathogenesis and management strategies. Drugs 2004;64:363-74. [Crossref] [PubMed]
- Chhabra A, Handa KK, Chakrabarti A, et al. Allergic fungal sinusitis: clinicopathological characteristics. Mycoses 1996;39:437-41. [Crossref] [PubMed]
- Corey JP, Delsupehe KG, Ferguson BJ. Allergic fungal sinusitis: allergic, infectious, or both? Otolaryngol Head Neck Surg 1995;113:110-9. [Crossref] [PubMed]


