L-BLP25 as a peptide vaccine therapy in non-small cell lung cancer: a review
Introduction
As one of the most prevalent malignancies worldwide, lung cancer is the leading cause of cancer-related death worldwide (1). There were 334,800 deaths due to lung cancer in 2006 in Europe (2) and 753,800 deaths in 2008 in Asia (3). The vast majority of cases (80-95%) are non-small cell lung cancer (NSCLC) (4). Patients with early-stage NSCLC may be cured by surgical resection, followed by adjuvant chemotherapy, which significantly improves the relapse-free and overall survival compared with surgery alone (5). However, a substantial proportion of patients with NSCLC are initially diagnosed with stage III disease (6).
Platinum-based chemotherapy together with concurrent thoracic radiotherapy is the first-line treatment for patients with unresectable stage IIIB NSCLC (4), but progress with this approach has reached an efficacy plateau, with few patients surviving beyond 5 years. Currently, attention has turned to whether incorporating consolidation or maintenance therapy into treatment regimens for stage III disease might improve the clinical outcome, but recent attempts to improve the outcome for stage III NSCLC patients have had limited success. Subsequent to the Southwest Oncology Group (SWOG) S9504 phase II study, which demonstrated the feasibility and tolerability of docetaxel as consolidation therapy following concurrent chemoradiotherapy in patients with stage IIIB disease (7), the potential of this approach was further investigated in a phase III study conducted by the Hoosier Oncology Group and US Oncology. However, it failed to improve survival, and even worse, it significantly increased toxicity (8). In another trial, maintenance therapy with gefitinib after concurrent chemoradiation therapy had a negative effect on survival in the SWOG 0023 trial (9).
Immunotherapy is a potential method for providing an improved therapeutic index by improving treatment tolerability (10). Modulation of the immune system, via vaccination or immunity checkpoint inhibition, has gained interest as a potential treatment pathway for NSCLC, particularly in view of successes with immunotherapy in melanoma and castration-resistant prostate cancer (11,12). The mucin 1 (MUC1) glycoprotein is overexpressed and abnormally glycosylated in NSCLC and other cancers (13,14). MUC1 promotes tumor cell growth, survival, and metastasis as a result of its high level of expression on the cell surface; the immunosuppressive properties of its released ectodomain; and its anti-adhesive properties, which prevent cell-cell adhesion and encourage metastasis (15,16). A number of factors make MUC1 a good target for immunotherapy, including high-level cell surface expression (17), antigenic epitopes (18) and aberrant glycosylation (19).
Rationale for immunotherapy with a MUC1 vaccine (L-BLP25)
L-BLP25 is a liposome-based vaccine consisting of a synthetic 25-amino acid lipopeptide derived from the tandem repeat region of MUC1, together with the nonspecific adjuvant monophosphoryl lipid A and three different lipids (15). Monophosphoryl lipid A serves as an adjuvant to induce a cellular immune response. The use of a liposome-based delivery system was intended to facilitate uptake of the antigenic peptide by antigen-presenting cells, such that lipopeptide is delivered into the intracellular space for presentation to Class I MHC. This presentation leads to an antigen-specific T-cell immune response that acts on MUC1-expressing tumors. Cytotoxic T lymphocytes specific to the MUC1 peptide sequence have been isolated from the peripheral blood or lymph nodes of adenocarcinoma patients with breast, pancreatic, and ovarian cancers (20-22). Stimulation of peripheral blood lymphocytes in vitro with L-BLP25 results in the generation of a strong MUC1-specific CTL response.
Cytotoxic T lymphocytes are specialized T lymphocytes that destroy cancer cells. T-cell receptors (TCRs) that are expressed on cytotoxic T cells can recognize a specific antigen. The antigen, which is often produced by cancer cells or viruses, can stimulate an immune response. A special antigen inside a cell is bound to a class I MHC molecule and brought to the surface of the cell by the class I MHC molecule, where it can be recognized by the T cell. If the TCR is specific for that antigen, it binds to the complex of the class I MHC molecules and the antigen. Then, the T cell destroys the cell.
Although the vaccine was designed to generate a primarily cell-mediated immune response, a humoral response may also be involved. Subgroups of breast cancer patients have been identified who have IgG antibodies specific to the MUC1 core peptide (23). Figure 1 shows the mechanism of action for L-BLP25 on MUC1.
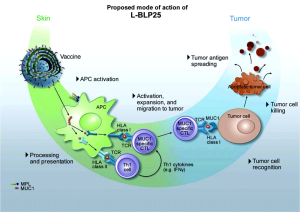
A single low dose of intravenous cyclophosphamide (300 mg/m2 to a maximum 600 mg) is administered prior to vaccination. MUC1 is highly immunosuppressive, and the low dose cyclophosphamide can overcome tolerance and enhance the effect of immunotherapy (24). Cyclophosphamide administered after immunization is immunosuppressive; thus, it is only given before the vaccination schedule is initiated. Cyclophosphamide plays a key role in the immunization strategy. Immune tolerance to self-antigens is a significant problem that must be overcome for many vaccine strategies to be effective (25). CD4+/25+ regulatory T cells are involved in the process of tumor-induced tolerance. Lutsiak et al. demonstrated that a single, low-dose parenteral administration of cyclophosphamide in female C57BL/6 mice leads to a decrease in both the number and functionality of T regs, enhancing apoptosis and the homeostatic proliferation of these cells (26). Similar findings have been noted using cyclophosphamide in a low dose, chronic daily dosing regimen, which is referred to as metronomic therapy (27).
As a MUC1 antigen specific immunotherapy, L-BLP25 induces a T-cell response to MUC1 in both a preclinical MUC1-transgenic lung cancer mouse model and patients (28-30), and preclinical studies have found that L-BLP25 is indeed capable of inducing a cellular immune response in mice (31). The 1-year survival rate is higher in patients with NSCLC who have high compared with low levels of natural MUC1 antibodies (32). Such observations provide the biological rationale, suggesting that inducing an anticancer immune response to MUC1 using a vaccination strategy might be an effective approach in the treatment of NSCLC.
In December 2013, Charles Butts and colleagues reported the results of the START trial (33), which was restricted to stage III NSCLC. The START trial is the first phase III trial of immunotherapy maintenance in patients with stage III NSCLC. Although the results did not show a survival improvement with tecemotide (consisting of the MUC1-derived 25-aminoacid BLP25 lipopeptide, the immunoadjuvant monophosphoryl lipid A, and three liposome-forming lipids) in all assigned patients, their data suggest that the subgroup of patients who received previous concurrent chemoradiotherapy might benefit from maintenance tecemotide.
To provide a foundation for ongoing and future clinical trials, a summary of the achievements gained in the completed clinical trials involving BLP25 administered as maintenance therapy for the treatment of unresectable stage III/IV NSCLC is necessary.
Preclinical study and phase I clinical trial
In preclinical murine studies, L-BLP25 induced a cellular immune response that is characterized by antigen-specific T-cell proliferation and the production of IFN-γ (31,34). Ultimately, this response led to early phase I and II clinical trials to assess its safety profile and efficacy. Last year, Wurz and his colleagues (35) evaluated the effects of L-BLP25 combined with low-dose cyclophosphamide pretreatment on Th1/Th2 cytokines using a novel human MUC1 transgenic (hMUC1.Tg) lung cancer mouse model. They found that the antitumor response to L-BLP25 requires at least two cycles and pre-treatment with cyclophosphamide. In addition, monitoring pro-inflammatory serum cytokines may be useful as a biomarker for the L-BLP25 response.
An initial phase I study in patients with NSCLC showed that the vaccine could be administered with minimal toxicity (36). Survival in the patients with advanced NSCLC who received L-BLP25 was sufficiently encouraging for proceeding with a phase II randomized study. In addition, an open-label, non-randomized phase I study combined with a double-blind, randomized, placebo-controlled phase II study was conducted in Japanese patients with unresectable stage III NSCLC after primary chemoradiotherapy. Their preliminary phase I safety data reported that L-BLP25 is well tolerated in Japanese patients, and the safety profile is consistent with that seen in previous studies.
Phase II clinical trials
Two foremost phase II trials established the dose and schedule of L-BLP25 and showed the ability of the vaccine to elicit a T-cell proliferative response (37). An open-label, randomized phase II trial in patients with stage IIIB or IV NSCLC who had underwent any first-line chemotherapy was undertaken to test the efficacy of L-BLP25. This trial recruited 171 patients from 17 centers in Canada and the United Kingdom. Patients were randomly assigned to either L-BLP25 plus the best supportive care (BSC) or the BSC alone. Patients in the L-BLP25 arm received a single intravenous injection dose of cyclophosphamide (300 mg/m2) followed by 8 consecutive weekly subcutaneous injections of L-BLP25 (1,000 μg). Subsequent immunizations were administered at 6-week intervals. The overall survival showed a trend toward longer survival with L-BLP25 plus the BSC vs. BSC alone [median: 17.4 vs. 13.0 months; adjusted hazard ratio (HR): 0.739, 95% CI: 0.509-1.073; P=0.112], with a post hoc subgroup analysis (n=65) suggesting a greater survival benefit in patients with stage IIIB locoregional disease. An updated analysis confirmed the survival benefit in this subgroup of patients (median: 30.6 months for L-BLP25 plus BSC vs. 13.3 months for BSC alone; adjusted HR: 0.548; 95% CI: 0.301-0.999) (38). No significant toxicity was reported in the L-BLP25 arm of this study; grade 1 flu-like symptoms and adverse events related to cyclophosphamide were the most frequent side effects. The quality of life (QoL) analysis revealed a clear advantage for the L-BLP25 arm over the BSC alone arm; more patients in the vaccine arm had clinically meaningful improvement or no change in the QoL, and more patients in the BSC arm had clinically meaningful worsening in the trial outcome index (28).
These safety findings are supported by a subgroup analysis of 16 patients who received L-BLP25 for at least 2 years (39). The safety of the new formulation of BLP25 has also been evaluated in a single-arm, multicenter open-label phase II study enrolling 22 patients, wherein there was a similar safety profile as for the original formulation (30). The results of this phase II study showed that maintenance therapy with L-BLP25 in patients with unresectable locoregional stage IIIB NSCLC is at least feasible and may prolong survival in this patient group.
Phase III clinical trials
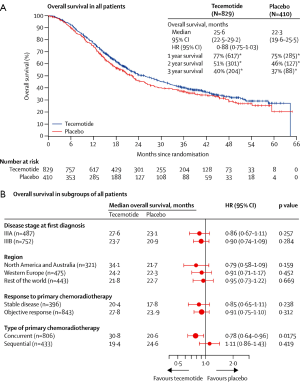
To determine whether the L-BLP25 vaccine enhances the survival of patients with stage III NSCLC who have received treatment with curative intent, Charles Butts and his colleagues started an international, randomized, double-blind phase III trial, which they called START (Stimulating Targeted Antigenic Response To NSCLC. In over 4 years of this trial, 1,513 patients (274 of them were excluded because of a clinical hold) with unresectable stage III NSCLC were enrolled. The patients had completed first-line treatment with chemoradiation, either concurrently or sequentially, and had stable disease or an objective clinical response. Randomly, 829 patients were assigned to receive tecemotide, and 410 were assigned to placebo on a double-blind basis at a 2:1 ratio after the modified intention-to-treat analysis. Incipiently, the study drug was given for 8 consecutive weekly subcutaneous injections of tecemotide (806 μg lipopeptide) or placebo, followed by an injection once every 6 weeks until disease progression. The primary endpoint was overall survival; however, the overall survival in patients who received tecemotide after chemoradiotherapy was not significantly different from those who received placebo [25.6 months (95% CI: 22.5-29.2) with tecemotide vs. 22.3 months (95% CI: 19.6-25.5) with placebo; HR: 0.88; 95% CI: 0.75-1.03; P=0.123]. Interestingly, subgroup analysis revealed that there was a remarkable improvement in the patients who received previous concurrent chemoradiotherapy. The median overall survival for 538 (65%) of the 829 patients assigned to tecemotide was 30.8 months (95% CI: 25.6-36.8) compared with 20.6 months (95% CI: 17.4-23.9) for the 268 (65%) of 410 patients assigned to placebo (adjusted HR: 0.78; 95% CI: 0.64-0.95; P=0.016). Currently, Charles Butts and his colleagues are planning a confirmatory randomized trial of tecemotide for patients with stage III NSCLC after concurrent chemoradiotherapy.
The biological rationale for such a difference in the response to tecemotide of NSCLC patients who previously received concurrent as opposed to sequential CRT remains unclear. A hypothesis was recently raised that the success of concurrent chemoradiotherapy in different solid tumors might be explained by the achievement of immunogenic cell death (40). Chiao-Jung Kao and his colleagues are trying to build preclinical animal models to accurately evaluate the effects of tecemotide in a preclinical setting. The mouse model may help explain the biological rationale for such a difference in the response to tecemotide of NSCLC patients who were previously receiving concurrent as opposed to sequential CRT (41).
There is an ongoing phase III trial, INSPIRE (Stimuvax Trial in Asian NSCLC Patients: Stimulating Immune Response), that is being conducted by Professor Wu to gain valuable insight into the potential role of L-BLP25 as maintenance therapy for East-Asian patients with unresectable stage III NSCLC. Currently, approximately 40 trial sites are contributing to the study to achieve a population of 420 patients. Figures 2 and 3 describe the overall survival from the phase III trial.

Conclusions
The theme within vaccine trials for NSCLC is a trend toward a benefit, but there is a general inability to achieve primary endpoints. However, in view of the results of clinical trials, it seems premature to think that targeting a single tumor-associated antigen is an invalid approach against NSCLC (42). The results of START should be considered a guide to understanding the interaction between antigen-specific immunotherapy and prior CRT. Numerous trials are underway internationally to determine whether novel immunotherapies can generate meaningful improvements in key clinical outcomes (such as the median overall survival and progression-free survival) in patients with lung cancer. Defining patient populations that will attain the greatest benefit to treatment with immunotherapeutics and determining the best time in a patient’s treatment course to administer immunotherapy remain open questions that require further exploration in phase III clinical trials.
One area for improvement in vaccine development is how to best design vaccines that generate both an immune response and a correlative clinical response. Perhaps the next phase of development should focus on the achievement of greater knowledge about the importance of MUC1 in NSCLC as well as on the identification of biomarkers that predict tecemotide efficacy (43). It may be important to monitor the immune response of cancer patients receiving immunotherapy over time and identify the parameters that correlate with survival. For example, it may be worthwhile to investigate an indicator of antigen-specific immune responses, such as the circulating levels of IFN-γ 24-48 h post-treatment, which might ensure that a given patient is at least exhibiting an immunological response. The trials should be conducted in prospectively defined subgroups in ongoing and future clinical trials. Efforts to enhance the ability of a vaccine to generate immune responses in a greater percentage of patients and to identify patient factors that predict a greater likelihood of achieving a measurable immune response are necessary for maximizing the vaccine immunotherapy’s ability to improve patient outcomes. The questions raised by START will hopefully be explained soon with substantial effort placed into the study of tecemotide.
Acknowledgements
Funding: This work was supported by the Natural Science Foundation of Jiangsu Province [grant number: BK2012482] and Jiangsu Provincial Special Program of Medical Science [grant number. BL2012030].
Disclosure: The authors declare no conflict of interest.
References
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893-917. [PubMed]
- Ferlay J, Autier P, Boniol M, et al. Estimates of the cancer incidence and mortality in Europe in 2006. Ann Oncol 2007;18:581-92. [PubMed]
- Chen W, Zhang S, Zou X. Evaluation on the incidence, mortality and tendency of lung cancer in China. Thoracic Cancer 2010;1:35-40.
- Crinò L, Weder W, van Meerbeeck J, et al. Early stage and locally advanced (non-metastatic) non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21 Suppl 5:v103-15. [PubMed]
- Winton T, Livingston R, Johnson D, et al. Vinorelbine plus cisplatin vs. observation in resected non-small-cell lung cancer. N Engl J Med 2005;352:2589-97. [PubMed]
- van Meerbeeck JP. Staging of non-small cell lung cancer: consensus, controversies and challenges. Lung Cancer 2001;34 Suppl 2:S95-107. [PubMed]
- Gandara DR, Chansky K, Albain KS, et al. Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol 2003;21:2004-10. [PubMed]
- Hanna N, Neubauer M, Yiannoutsos C, et al. Phase III study of cisplatin, etoposide, and concurrent chest radiation with or without consolidation docetaxel in patients with inoperable stage III non-small-cell lung cancer: the Hoosier Oncology Group and U.S. Oncology. J Clin Oncol 2008;26:5755-60. [PubMed]
- Kelly K, Chansky K, Gaspar LE, et al. Phase III trial of maintenance gefitinib or placebo after concurrent chemoradiotherapy and docetaxel consolidation in inoperable stage III non-small-cell lung cancer: SWOG S0023. J Clin Oncol 2008;26:2450-6. [PubMed]
- Bradbury PA, Shepherd FA. Immunotherapy for lung cancer. J Thorac Oncol 2008;3:S164-70. [PubMed]
- Forde PM, Reiss KA, Zeidan AM, et al. What lies within: novel strategies in immunotherapy for non-small cell lung cancer. Oncologist 2013;18:1203-13. [PubMed]
- Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control 2013;20:22-31. [PubMed]
- Bafna S, Kaur S, Batra SK. Membrane-bound mucins: the mechanistic basis for alterations in the growth and survival of cancer cells. Oncogene 2010;29:2893-904. [PubMed]
- Raina D, Kosugi M, Ahmad R, et al. Dependence on the MUC1-C oncoprotein in non-small cell lung cancer cells. Mol Cancer Ther 2011;10:806-16. [PubMed]
- Sangha R, Butts C. L-BLP25: a peptide vaccine strategy in non small cell lung cancer. Clin Cancer Res 2007;13:s4652-4. [PubMed]
- Tang CK, Apostolopoulos V. Strategies used for MUC1 immunotherapy: preclinical studies. Expert Rev Vaccines 2008;7:951-62. [PubMed]
- Apostolopoulos V, Pietersz GA, McKenzie IF. MUC1 and breast cancer. Curr Opin Mol Ther 1999;1:98-103. [PubMed]
- Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res 1993;53:641-51. [PubMed]
- Hull SR, Bright A, Carraway KL, et al. Oligosaccharide differences in the DF3 sialomucin antigen from normal human milk and the BT-20 human breast carcinoma cell line. Cancer Commun 1989;1:261-7. [PubMed]
- Jerome KR, Barnd DL, Bendt KM, et al. Cytotoxic T-lymphocytes derived from patients with breast adenocarcinoma recognize an epitope present on the protein core of a mucin molecule preferentially expressed by malignant cells. Cancer Res 1991;51:2908-16. [PubMed]
- Berd D, Maguire HC Jr, McCue P, et al. Treatment of metastatic melanoma with an autologous tumor-cell vaccine: clinical and immunologic results in 64 patients. J Clin Oncol 1990;8:1858-67. [PubMed]
- Ioannides CG, Fisk B, Jerome KR, et al. Cytotoxic T cells from ovarian malignant tumors can recognize polymorphic epithelial mucin core peptides. J Immunol 1993;151:3693-703. [PubMed]
- Jerome KR, Domenech N, Finn OJ. Tumor-specific cytotoxic T cell clones from patients with breast and pancreatic adenocarcinoma recognize EBV-immortalized B cells transfected with polymorphic epithelial mucin complementary DNA. J Immunol 1993;151:1654-62. [PubMed]
- Mastrangelo MJ, Berd D, Maguire H Jr. The immunoaugmenting effects of cancer chemotherapeutic agents. Semin Oncol 1986;13:186-94. [PubMed]
- Ko HJ, Kim YJ, Kim YS, et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res 2007;67:7477-86. [PubMed]
- Lutsiak ME, Semnani RT, De Pascalis R, et al. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 2005;105:2862-8. [PubMed]
- Ghiringhelli F, Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007;56:641-8. [PubMed]
- Butts C, Murray N, Maksymiuk A, et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J Clin Oncol 2005;23:6674-81. [PubMed]
- Butts C, Maksymiuk A, Goss G, et al. Updated survival analysis in patients with stage IIIB or IV non-small-cell lung cancer receiving BLP25 liposome vaccine (L-BLP25): phase IIB randomized, multicenter, open-label trial. J Cancer Res Clin Oncol 2011;137:1337-42. [PubMed]
- Butts C, Murray RN, Smith CJ, et al. A multicenter open-label study to assess the safety of a new formulation of BLP25 liposome vaccine in patients with unresectable stage III non-small-cell lung cancer. Clin Lung Cancer 2010;11:391-5. [PubMed]
- Guan HH, Budzynski W, Koganty RR, et al. Liposomal formulations of synthetic MUC1 peptides: effects of encapsulation versus surface display of peptides on immune responses. Bioconjug Chem 1998;9:451-8. [PubMed]
- Hirasawa Y, Kohno N, Yokoyama A, et al. Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am J Respir Crit Care Med 2000;161:589-94. [PubMed]
- Butts C, Socinski MA, Mitchell PL, et al. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol 2014;15:59-68. [PubMed]
- Agrawal B, Krantz MJ, Reddish MA, et al. Rapid induction of primary human CD4+ and CD8+ T cell responses against cancer-associated MUC1 peptide epitopes. Int Immunol 1998;10:1907-16. [PubMed]
- Wurz GT, Gutierrez AM, Greenberg BE, et al. Antitumor effects of L-BLP25 Antigen-Specific tumor immunotherapy in a novel human MUC1 transgenic lung cancer mouse model. J Transl Med 2013;11:64. [PubMed]
- Palmer M, Parker J, Modi S, et al. Phase I study of the BLP25 (MUC1 peptide) liposomal vaccine for active specific immunotherapy in stage IIIB/IV non-small-cell lung cancer. Clin Lung Cancer 2001;3:49-57; discussion 58. [PubMed]
- North S, Butts C. Vaccination with BLP25 liposome vaccine to treat non-small cell lung and prostate cancers. Expert Rev Vaccines 2005;4:249-57. [PubMed]
- Butts C, Maksymiuk A, Goss G, et al. A multi-centre phase IIB randomized controlled study of BLP25 liposome vaccine (L-BLP25 or Stimuvax) for active specific immunotherapy of non-small cell lung cancer (NSCLC): updated survival analysis. J Thorac Oncol 2007;2:s332-3.
- Butts C, Anderson H, Maksymiuk A, et al. Long-term safety of BLP25 liposome vaccine (L-BLP25) in patients (pts) with stage IIIB/IV non-small cell lung cancer (NSCLC). J Clin Oncol 2009;27:3055.
- Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst 2013;105:256-65. [PubMed]
- Kao CJ, Wurz GT, Schröder A, et al. Clarifying the pharmacodynamics of tecemotide (L-BLP25)-based combination therapy. Oncoimmunology 2013;2:e26285. [PubMed]
- Kroemer G, Zitvogel L, Galluzzi L. Victories and deceptions in tumor immunology: Stimuvax®. Oncoimmunology 2013;2:e23687. [PubMed]
- Situ D, Wang J, Ma Y, et al. Expression and prognostic relevance of MUC1 in stage IB non-small cell lung cancer. Med Oncol 2011;28 Suppl 1:S596-604. [PubMed]


