Non-intubated video-assisted thoracoscopic surgery vs. intubated video-assisted thoracoscopic surgery for thoracic disease: a systematic review and meta-analysis of 1,684 cases
Introduction
Video-assisted thoracoscopic surgery (VATS), a standard treatment for early lung cancer (1-4), solitary pulmonary nodule (5-8) and hyperhidrosis (9), has been found to be associated with fewer complications as compared with traditional open thoracotomy. However, intubated VATS (IVTAS) under general anesthesia has been shown to be associated with several unfavorable side effects, such as ventilation-related lung injury, intubation-induced airway injury, postoperative vomiting and nausea (10). Since the first case report by Jacobeus and Bethune in 1922 (11), non-intubated video-assisted thoracoscopic surgery (NIVATS) under local anesthesia has gradually emerged as a promising technique for thoracic surgery. Since then, a series of clinical trials have been launched to explore the feasibility of NIVATS under local or regional anesthesia, including thoracic epidural anesthesia and intercostal-nerve blocks in general.
Chen et al. (12) report that lung cancer patients undergoing non-intubated surgery have lower complication rates, lower occurrence of sore throat, earlier postoperative oral intake, but have no remarkable differences in postoperative complication rate and hospital stays. Despite many studies on the comparison between NIVATS and IVATS have been reported, the majority of them have limited samples with conflicting results. We performed a systematic review and meta-analysis on pooled data from eligible studies to compare efficacy and safety between NIVATS and IVATS on.
Methods
Anesthesia procedure
The anesthesia procedure is the comprehensive procedure from the literature (13-16). During the operation procedure, all patients were continuously monitored by electrocardiogram, pulse oxymeter, blood pressure, body temperature, and end-tidal CO2 by insertion of a detector into the nostril. In the non-intubated or awake group, patients underwent the thoracic epidural anesthesia (TEA) or intercostal nerve block. The intercostal nerve block was usually carried out by local injection of lidocaine 2% (4 mg/kg) and ropivacaine 7.5% (2 mg/kg). The objective of TEA was to achieve somatosensory and motor block at the T1–T8 levels and preserve diaphragmatic respiration. After premedication with midazolam, a thoracic epidural catheter was inserted at the T4 level. In the operating room, patients received a continuous infusion of sufentanil (1.66 µg/mL) and ropivacaine (0.2–0.5%) or 2% lidocaine into the epidural space. Meanwhile, patients breathed O2 through a venturi mask to maintain oxygen saturation greater than 90%.
General anesthesia was induced by intravenous propofol (1.5 to 2.0 mg/kg), fentanyl (0.1 mg), vecuronium (0.1 mg/kg) or rocuronium (0.6 mg/kg). A left-sided double-lumen tube was routinely inserted. The epidural catheter was removed at 48 hours after surgery.
Literature search strategy
This meta-analysis was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (17). A systematic and comprehensive computerized literature search was performed in PubMed, ISI Web of Science and Cochrane Library using a combination of MeSH terms “non-intubated”, “non-tracheal intubation”, “awake”, “video-assisted thoracoscopic surgery”, “VATS”, “thoracoscopic” and “thoracoscopy”, to screen literature up to February 2018. The relevant papers were subsequently searched as a supplement.
Criteria for inclusion and exclusion
Eligible studies included the following criteria: (I) randomized controlled trials (RCTs) or observational studies that compared non-intubated or awake VATS under local or regional anesthesia with intubated VATS under general anesthesia in patients for thoracic surgery; (II) studies with sufficient data for estimation of weighted mean differences (WMDs) or odds ratios (OR); (III) studies in which both groups of patients in a study underwent the same surgical procedures for VATS; (IV) the most recent study was selected in case of duplication. The exclusion criteria were as follows: (I) absence of comparison of non-intubated VATS with intubated VATS for thoracic surgery; (II) patients in both groups received different surgical procedures; (III) reviews, letters, editorials, expert opinions, case report, and animal experiments; (IV) relevant data could not be extracted.
Data extraction
Two of the authors (K Zhang and HG Chen) independently performed the data extraction from the eligible studies according to a standard protocol. If data were missing or incomplete in their publications, we contacted the authors by email. Disagreements were resolved by consensus with a senior investigator (J Zhang). The extracted data included the first author, year of publication, study design, number of study subjects, postoperative complications, global in-operating time, operating time, anesthesia time, hospital stay, perioperative mortality, estimated blood loss (EBL), chest-tube placement time, visual analogue scale (VAS) score, anesthesia satisfaction score (ASS) and matching criteria. Postoperative complications were as follows: VAS score is a unidimensional measure of pain intensity, and ASS score is a measure of satisfaction with anesthesia delivery. The postoperative complications include hoarseness, hemothorax, cardiac complications (arrhythmia, atrial fibrillation, and cardiac failure), lung complications (air leaks >5 days, pulmonary infection, atelectasis, respiratory failure), and death.
Quality assessment of included studies
The quality of the included RCTs was assessed by the tool “risk of bias” according to the Cochrane Handbook (version 5.3) (18), and the Jadad scale, which consisted of randomization (0–2 points), blinding (0–2 points) and withdrawals (0–1 point). Studies scored ≥3 points were defined as high quality. The quality of all observational studies was assessed by the Newcastle-Ottawa Scale (NOS) (19,20), based on three factors: patient selection, comparability of the study groups and exposure. A rating of 0-9 was allocated to each study based on the above three parameters and a study with a score ≥6 was considered as high-quality and a score lower than 6 was defined as poor quality.
Statistical analysis
The data were analyzed using Review Manager software (version 5.3, Cochrane Collaboration, UK). WMDs with 95% confidence intervals (CIs) was used to analyze the continuous variables and dichotomous variables were calculated using OR. Heterogeneity was assessed by I2 statistics. A random-effects model was adopted if high between-study heterogeneity (P<0.1 or I2>50%) was observed; otherwise, a fixed-effects model was used. Funnel plots were used to estimate potential publication bias, and asymmetry of the funnel plot was tested by Begg’s test and Egger’s test (21). A two-tailed P value of 0.05 or less was deemed statistically significant.
Results
Studies characteristics
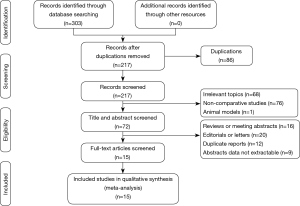
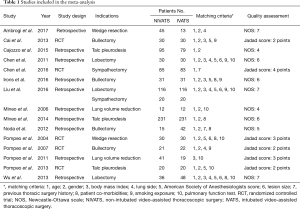
A flow diagram depicting the selection of eligible studies according to the PRISMA guidelines is shown in Figure 1 After the exclusion of 288 studies that did not meet our inclusion criteria, 15 eligible studies (12,15,22-34) were screened out for meta-analyses. One study was published in Chinese, and 14 studies were published in English. As shown in Table 1, 9 studies were of good quality based on the Newcastle-Ottawa Scale and Jadad scales. Quality of included studies was generally low. Two of 5 RCTs identified the methods for randomization (12,31), and the rest did not provide detailed methods for randomization (23,30,31). Only Chen et al. used sequentially numbered sealed envelopes disclosing the type of procedure to make the allocation. Ten studies were retrospective case-control studies and 5 studies were RCT studies. One study (34) includes 2 kinds of surgery: lobectomy and sympathectomy. A total of 1,684 patients were included, of which 858 (51.0%) patients underwent non-intubated VATS and 826 (49.0%) patients underwent intubated VATS. The details of all the included studies were summarized in Table 1.
Full table
Sensitivity analysis and publication bias
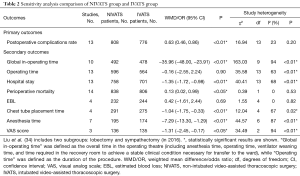
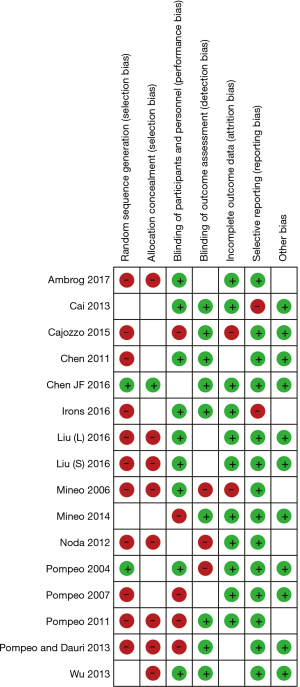
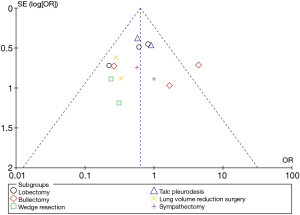
The sensitivity analysis was performed to evaluate the stability of this meta-analysis, the results showed that our methods were reliable and did not remarkably change the primary outcome of overall analysis (Table 2). Publication bias was tested by Begg’s test and Egger’s test which indicated that all included studies were within the 95% CIs, with evidence of symmetry, with no significant publication bias (Begg’s test and Egger’s test both P>0.05). Figure 2 shows the risk of bias summary. The majority assessment of selected studies was of low risk. The funnel plot (Figure 3) of the primary outcome (postoperative complication) also indicates that the publication bias of this study was small and acceptable.
Full table
Primary outcome
Postoperative complications
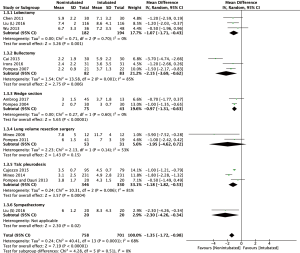
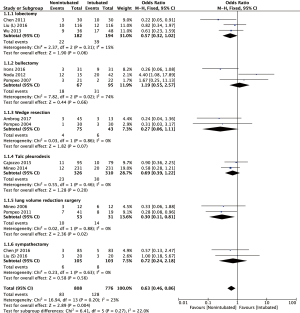
The Forrest plot of postoperative complications between the NIVATS group and the IVATS group is shown in Figure 4. Thirteen studies (12,15,22,24-29,31-34) with complete postoperative complication data were included in the analysis. As the heterogeneity between studies was acceptable (I2 statistic =23%, P=0.20), the fixed-effect model was adopted for this meta-analysis. The result showed that complication rate of NIVATS group was significantly lower (10.3% and 16.5%; OR: 0.63; 95% CI, 0.46–0.86; P=0.004) as compared with the intubated group.
Secondary outcomes
Global in-operating time, operating time and anesthesia time
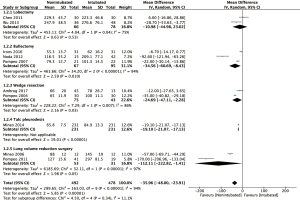
Analysis of data from 10 studies (15,22,25-29,31-33) revealed a significantly shorter global in-operating time in the non-intubated group than the intubated VATS group (WMD: −35.96 min; 95% CI, −48.00 to −23.91; P<0.00001; Figure 5). However, analysis of operating time data from thirteen studies (12,15,22-25,27,29-34) with 1,160 patients showed no difference between the two groups (WMD: −0.16 min; 95% CI, −2.55 to 2.24; P=0.90). In the NIVATS group, the anesthesia time was significantly shorter than the control group (WMD: −7.29 min; 95% CI, −13.30 to −1.29; P=0.02; Figure 6), which may be attributed to a shorter time of induction of anesthesia and endotracheal intubation.

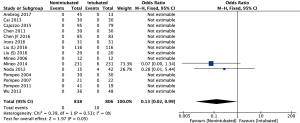
Hospital stay and perioperative mortality
Thirteen studies reported for the duration of hospitalization. There was significant heterogeneity among these studies (I2 statistic =68%, P<0.01). The meta-analysis showed a significantly reduced hospital stay in the NIVATS group (WMD: −1.35 days; 95% CI, −1.72 to −0.98; P<0.00001). The forest plot is summarized in Figure 7. Fourteen included studies (12,15,22-29,31-34) listed the perioperative mortality rate out of which twelve reported no mortality. The perioperative morbidity rate was lower in the non-intubated VATS cases than in the intubated VATS cases (0% vs. 1.2%; OR =0.13; 95% CI, 0.02–0.99; P=0.05, Figure 8).
EBL and chest-tube placement time
Only four studies (15,23,25,34) with 476 patients evaluated EBL, which showed no significant difference between the NIVATS and IVATS groups (WMD: 0.42 mL; 95% CI, −1.61 to −2.44; P=0.69). The duration of chest-tube placement time reported, however, was shorter in the NIVATS group compared with the IVATS group (WMD: −1.04 days; 95% CI, −1.75 to −0.33; P<0.01).
Visual analogue scale (VAS) score and anesthesia satisfaction score (ASS)
The present meta-analysis included three studies with complete data on VAS score (12,31,32). Due to the heterogeneity of studies (I2 statistic =94%, P<0.01), a random effect model was adopted. The result of WMD =−1.31 (95% CI, −2.45 to −0.17) indicated a lower postoperative pain in NIVATS group as compared with the control group (P=0.02). Three studies evaluated the anesthesia satisfaction score comprising four grades (4, excellent; 3, good; 2, satisfactory; 1, unsatisfactory) (31-33). The analysis revealed no significant difference in ASS between the two groups (WMD: 0.54, 95%CI, −0.11 to 1.19, P=0.11).
Subgroup analysis
Outcomes included at least in two studies were further evaluated by subgroup analysis.
NIVATS lobectomy vs. IVATS lobectomy
There were no significant differences in the subgroup analysis as compared with the original analysis, except for the hospital stay (WMD: −1.07; 95% CI, −1.71 to −0.43; P<0.01) and the global-in operating time (WMD: −10.98; 95% CI, −44.98 to 23.02; P=0.53).
NIVATS bullectomy vs. IVATS bullectomy
There were no significant differences in the subgroup analysis as compared with the original analysis in hospital stay (WMD: −2.15; 95% CI, −3.69 to −0.62; P<0.01) and anesthesia time (WMD: −8.57; 95% CI, −14.80 to −2.34; P<0.01). However, we found no significant difference between two groups in the incidence of postoperative complications (26.9% vs. 32.6%; OR =1.19, 95% CI, 0.55–2.57; P=0.66).
NIVATS wedge resection vs. IVATS wedge resection
Subgroup analysis in anesthesia time was not performed due to the lack of sufficient data. There were no significant differences in the subgroup analysis compared with the original analysis in hospital stay (WMD: −0.97 days; 95% CI, −1.31 to −0.63; P<0.01) or the frequency of postoperative complications (5.3% vs. 14%; OR =0.27, 95% CI, 0.06–1.11; P=0.07).
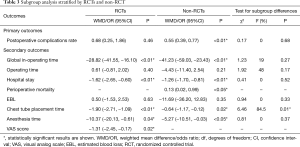
RCTs vs. non-RCTs
As shown in Table 3, the results and subgroup differences were compared between RCT and non-RCT studies. Most significant results were consistent with overall significances in the original analysis. Except for chest tube placement time (P=0.01), the other outcomes had no significant subgroup differences between RCTs and non-RCTs (all P>0.05).
Full table
Discussion
Intubated anesthesia with single-lung mechanical ventilation remains the standard approach for thoracic surgery. However, this anesthesia method is closely associated with some adverse effects (10). In this decade, the non-intubated technique has been gradually applied in general thoracic operations, which has been reported to decrease postoperative complications (12). Although NIVATS is associated with many benefits as compared with IVATS, whether NIVATS could achieve safer and more efficient in patients with thoracic disease remains controversial. In this study, the meta-analysis comprising 5 RCTs and 10 retrospective studies and 1,684 patients revealed that NIVATS under regional or local anesthesia could be safer and more efficient for thoracic disease as compared with IVATS, in term of postoperative complications, hospital stays, anesthesia and chest intubation time, postoperative pain and perioperative mortality rate.
The NIVATS procedure is associated with minimal trauma and quick recovery, and low rate of postoperative complications, such as pneumonia, air leak, atrial fibrillation, hoarseness, and gastrointestinal vomiting. We believe that some complications in the IVATS might be due to the endotracheal intubation, especially for double-lumen tube intubation. Although NIVATS is more challenging for surgeons compared with IVATS procedure, our meta-analysis in postoperative complications reveals that the NIVATS approach is safe for thoracic surgery than IVATS (10.3% vs. 16.5%, P<0.01). In addition, patients undergoing NIVATS can breathe spontaneously without mechanical ventilation, which eliminates intubation-related complications and side effects of general anesthesia. Moreover, it has been demonstrated that NIVATS under local or regional anesthesia inhibits the level of inflammatory cytokines (tumor necrosis factor-α and C-reactive protein) (35), lymphocyte activity (36) and surgical stress hormones response (37) as compared with IVATS under general anesthesia. These may account for the lower incidence of postoperative complications and hospital stays in those treated with NIVATS.
In this study, the NIVATS group had a shorter hospital stay, less anesthesia time, less chest-tube placement time and less chest pain as compared with the IVATS group. However, there were no differences in operating time and blood loss between groups. These results suggest that NIVATS is a better anesthesia procedure than the IVATS method. Nevertheless, our findings are inconsistent with Wu et al.’s report (15), in which no significant differences were found in blood loss, postoperative hospital stay and complication rate between NIVATS and IVATS methods. This discrepancy suggests that more randomized-controlled trials should be conducted to investigate this issue.
To minimize the publication bias, we conducted a sensitivity analysis. Sensitivity analysis showed that removing any study from the pooled data did not vary the original results substantially. Although our meta-analysis was not from pure RCTs and without randomization in most of the included RCTs, Begg’s test and Egger’s test did not show any publication bias. Nevertheless, due to only half of these studies were of high quality, these results should be interpreted with caution.
As shown in Table 2, the between-study heterogeneity was significant for the majority of continuous variables, but not significant for dichotomous variables. Although the surgical method was one of the main reasons for between-study heterogeneity, other factors still cannot be ruled out, such as expertise level of the surgeon or anesthetist’s skill level, VATS devices, matching criteria and measurement standards. The random-effects model can reduce but not completely eliminate the between-study heterogeneity.
Some limitations of our study should be pointed out. Firstly, most of the included studies were retrospective studies without sufficient information about randomization and blinding. Secondly, most studies were conducted in major medical centers with varying protocols and surgeons with different level of expertise, which might not well reflect the general situation. Further systematic reviews should be conducted to evaluate different indications separately when enough literature is available. Moreover, there was significant heterogeneity in the global in-operating time, operating time, anesthesia time, ASS and VAS scores among the included studies. Sensitivity analysis significantly reduced the heterogeneity for operating time and ASS, but not for the other factors. Except for surgical procedures, differences in lesion size, lesion location, properties of the lesion, patients’ physical status or nationality are likely to contribute to the high heterogeneity. Lastly, because this procedure has only been used clinically in recent years, the lack of long-term follow-up studies may bias the results of our meta-analysis.
Conclusions
This study showed that non-intubated VATS could reduce the rate of postoperative complications, shorten hospital stay and decrease perioperative mortality rate, indicating that the non-intubated VATS is a safe, effective and feasible technique for the thoracic disease. However, the long-term efficacy of non-intubated VATS remains to be investigated. A well-designed, large-scale, multi-center RCT is needed to further validate its advantages.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Onaitis MW, Petersen RP, Balderson SS, et al. Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 2006;244:420-5. [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Rueth NM, Andrade RS. Is VATS lobectomy better: perioperatively, biologically and oncologically? Ann Thorac Surg 2010;89:S2107-11. [Crossref] [PubMed]
- Allen MS, Deschamps C, Lee RE, et al. Video-assisted thoracoscopic stapled wedge excision for indeterminate pulmonary nodules. J Thorac Cardiovasc Surg 1993;106:1048-52. [PubMed]
- Mitruka S, Landreneau RJ, Mack MJ, et al. Diagnosing the indeterminate pulmonary nodule: percutaneous biopsy versus thoracoscopy. Surgery 1995;118:676-84. [Crossref] [PubMed]
- Hazelrigg SR, Magee MJ, Cetindag IB. Video-assisted thoracic surgery for diagnosis of the solitary lung nodule. Chest Surg Clin N Am 1998;8:763-74. vii. [PubMed]
- Murasugi M, Onuki T, Ikeda T, et al. The role of video-assisted thoracoscopic surgery in the diagnosis of the small peripheral pulmonary nodule. Surg Endosc 2001;15:734-6. [Crossref] [PubMed]
- Cerfolio RJ, De Campos JR, Bryant AS, et al. The Society of Thoracic Surgeons expert consensus for the surgical treatment of hyperhidrosis. Ann Thorac Surg 2011;91:1642-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Bonome C, Fieira E, et al. Non-intubated video-assisted thoracoscopic lung resections: the future of thoracic surgery? Eur J Cardiothorac Surg 2016;49:721-31. [Crossref] [PubMed]
- HC J. The practical importance of thoracoscopy in surgery of the chest. Surg Gynecol Obstet 1922.289-96.
- Chen J, Du Q, Lin M, et al. Transareolar Single-Port Needlescopic Thoracic Sympathectomy Under Intravenous Anesthesia Without Intubation: A Randomized Controlled Trial. J Laparoendosc Adv Surg Tech A 2016;26:958-64. [Crossref] [PubMed]
- Al-Abdullatief M, Wahood A, Al-Shirawi N, et al. Awake anaesthesia for major thoracic surgical procedures: an observational study. Eur J Cardiothorac Surg 2007;32:346-50. [Crossref] [PubMed]
- Chen KC, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lung resection: a 3-year experience with 285 cases in a single institution. J Thorac Dis 2012;4:347-51. [PubMed]
- Wu CY, Chen JS, Lin YS, et al. Feasibility and safety of nonintubated thoracoscopic lobectomy for geriatric lung cancer patients. Ann Thorac Surg 2013;95:405-11. [Crossref] [PubMed]
- Wang ML, Hung MH, Chan KC, et al. Intraoperative multiple intercostal nerve blocks exert anesthetic-sparing effect: A retrospective study on the effect-site concentration of propofol infusion in nonintubated thoracoscopic surgery. Acta Anaesthesiol Taiwan 2016;54:77-80. [Crossref] [PubMed]
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010;8:336-41. [Crossref] [PubMed]
- Higgins JPT, Churchill R, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration, 2011. Hoboken, New Jersey: John Wiley & Sons, Ltd., 2011.
- Wells GS, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of non randomised studies in meta-analyses. Ottawa Hospital Research Institute. Available online: http://wwwohrica/programs/clinical_epidemiology/oxfordasp
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. [Crossref] [PubMed]
- Peters JL, Sutton AJ, Jones DR, et al. Comparison of two methods to detect publication bias in meta-analysis. JAMA 2006;295:676-80. [Crossref] [PubMed]
- Ambrogi V, Sellitri F, Perroni G, et al. Uniportal video-assisted thoracic surgery colorectal lung metastasectomy in non-intubated anesthesia. J Thorac Dis 2017;9:254-61. [Crossref] [PubMed]
- Cai K, Wang X, Ye J, et al. Laryngeal mask anesthesia in video-assisted thoracoscopic surgery for pulmonary bulla: comparison with intubation anesthesia. Nan Fang Yi Ke Da Xue Xue Bao 2013;33:756-60. [PubMed]
- Cajozzo M, Lo Iacono G, Raffaele F, et al. Thoracoscopy in pleural effusion--two techniques: awake single-access video-assisted thoracic surgery versus 2-ports video-assisted thoracic surgery under general anesthesia. Future Oncol 2015;11:39-41. [Crossref] [PubMed]
- Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg 2011;254:1038-43. [Crossref] [PubMed]
- Irons JF, Miles LF, Joshi KR, et al. Intubated Versus Nonintubated General Anesthesia for Video-Assisted Thoracoscopic Surgery-A Case-Control Study. J Cardiothorac Vasc Anesth 2017;31:411-7. [Crossref] [PubMed]
- Mineo TC, Pompeo E, Mineo D, et al. Awake nonresectional lung volume reduction surgery. Annals of Surgery 2006;243:131-6. [Crossref] [PubMed]
- Mineo TC, Sellitri F, Tacconi F, et al. Quality of life and outcomes after nonincubated versus intubated video-thoracoscopic pleurodesis for malignant pleural effusion: comparison by a case-matched study. J Palliat Med 2014;17:761-8. [Crossref] [PubMed]
- Noda M, Okada Y, Maeda S, et al. Is there a benefit of awake thoracoscopic surgery in patients with secondary spontaneous pneumothorax? J Thorac Cardiovasc Surg 2012;143:613-6. [Crossref] [PubMed]
- Pompeo E, Dauri M. Is there any benefit in using awake anesthesia with thoracic epidural in thoracoscopic talc pleurodesis? J Thorac Cardiovasc Surg 2013;146:495-7.e1. [Crossref] [PubMed]
- Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg 2004;78:1761-8. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo D, et al. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J Thorac Cardiovasc Surg 2007;133:786-90. [Crossref] [PubMed]
- Pompeo E, Tacconi F, Mineo TC. Comparative results of non-resectional lung volume reduction performed by awake or non-awake anesthesia. Eur J Cardiothorac Surg 2011;39:e51-8. [Crossref] [PubMed]
- Liu J, Cui F, Pompeo E, et al. The impact of non-intubated versus intubated anaesthesia on early outcomes of video-assisted thoracoscopic anatomical resection in non-small-cell lung cancer: a propensity scores matching analysis. Eur J Cardiothorac Surg 2016;50:920-5. [Crossref] [PubMed]
- Liu J, Cui F, Li S, et al. Nonincubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: a randomized control study. Surg Innov 2015;22:123-30. [Crossref] [PubMed]
- Vanni G, Tacconi F, Sellitri F, et al. Impact of awake video thoracoscopic surgery on postoperative lymphocyte responses. Ann Thorac Surg 2010;90:973-8. [Crossref] [PubMed]
- Tacconi F, Pompeo E, Sellitri F, et al. Surgical stress hormones response is reduced after awake video thoracoscopy. Interact Cardiovasc Thorac Surg 2010;10:666-71. [Crossref] [PubMed]