
Association between cardiovascular risk factors and the diameter of the main pulmonary artery in asymptomatic population in the Appalachian region
Introduction
Pulmonary artery (PA) diameter may be altered in association with cardiovascular (CV) risk factors as noted in aorta in systemic hypertension. Alterations in elastic properties of the PA have been documented in pulmonary hypertension (PH) in animal (1-4) and in human studies (2,5-8). The flow of blood from the right ventricle to the PA and all the way to the capillary level depends on the resistance and compliance of the PA, which are the fundamental conduit for maintenance of the right heart hemodynamics (9). Increase in PA stiffness leads to increased resistance and decreased compliance causing more vascular damage to smaller pulmonary vessels (3,4,7,10).
Several studies showed that large PA diameter as assessed by Computed Tomography (CT) (11-18) or magnetic resonance imaging (MRI) (19-21) may be used to detect PH early, and smaller PA diameter makes the presence of PH unlikely. However, smaller PA diameter on imaging studies does not exclude the presence of PH (22). Nonetheless, PH is just one of the many causes of a dilated PA, and with the increasing use of noninvasive imaging, physicians are more likely to observe incidental findings of PA enlargement.
Studies done in this area focused on the imaging criteria for diagnosis of PA dilatation and addressed the association with PH and/or investigated the ability of a dilated PA to predict the presence of PH (23). Rarely, left main coronary artery compression in PH or in various congenital heart diseases due to enlarged PA has been described (24-31). Non-invasive measurement of pulmonary trunk diameter may be helpful in determining the likelihood of left coronary artery compression and in selecting patients for diagnostic angiography for evaluation of chest pain (11). From a clinical aspect, studies showed the loss of compliance in the PA to be a predictor for increased mortality in patients who have PH (23,32,33). However, the effects of CV risk factors on the diameter of the main pulmonary artery (MPA) were not fully studied. Therefore, the objective of this study is to determine the association between CV risk factors and the MPA diameter.
Methods
Study population
The study population comprised of asymptomatic individuals with no known CV diseases from the Central Appalachian region of Kentucky, North Carolina, Tennessee and Virginia. The study population participated in a coronary artery calcification (CAC) screening program in Wellmont Heart Institute (now Ballad Health) through either self-referral or referral from a physician. Adults aged 18 years or older were eligible for the screening if they were referred by a physician. For self-referral, only males aged ≥45 years and females aged ≥55 years were eligible.
The eligible individuals who agreed to participate were asked to sign an informed consent before the study was conducted. All participants were required to complete a short questionnaire on demographics (age, sex, and race/ethnicity), health behaviors (smoking and sedentary lifestyle), medical conditions (diabetes, hypertension, hypercholesterolemia, history of coronary artery disease (CAD) and family history of CAD). This study was approved by the Institutional Review Board of the collaborating health system and the authors’ institution.
There were 1,605 individuals who participated in the screening program from January 2014 to December 2016. Non-whites (n=20) and participants with no information on race (n=25) were excluded. Further, individuals with missing information in any of the dependent or independent variables (n=276) were excluded from the sample. In addition, individuals who had extreme recorded values of body mass index (BMI) (BMI <14 kg/m2) (n=1) and MPA diameter (diameter>50 mm) (n=1) were excluded from the study to reduce the effect of outliers in the study results. In total, 1,282 participants were included in the final analyses.
Dependent variable
Following standard procedure and protocol (34) for CT assessment of MPA (Figure 1), individuals are required to remove all metal objects from the chest area and three stick-on electrode leads are placed on the chest. The person then lies still on a 64 slices high resolution CT scanner as it advanced through the donut-shaped scanner. The scanner passes over the person for three times, with the person holding his/her breathe for ≤10 seconds for each time. Then during interpretation, the MPA and aortic diameter was measured.
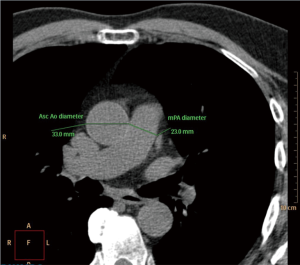
Independent variables
Information on demographics (age and sex) and CV risk factors were obtained from the self-administered questionnaire. Age was recorded as a continuous variable. For descriptive analyses, two categories of age (age ≤60 years and >60 years) were created. The self-reported height and weight measurements were used to calculate the BMI. For descriptive analyses, participants with BMI ≥30 kg/m2 were classified as ‘obese’ and the rest were classified as ‘not obese’. However, during regression analyses, BMI was used as a continuous variable. The information on clinical status of diabetes, hypertension, and hypercholesterolemia were collected by asking the participants if they had ever been informed by a physician about whether they had diabetes, hypertension or hypercholesterolemia and/or were using any medication to treat these conditions. The responses were recorded as a dichotomous variable (yes/no). Participants also reported whether they were current smokers, former smokers, or never smokers. During analyses, the participants who reported themselves as former and current smokers were classified as ‘ever smokers’ and those who have never smoked were classified as ‘never smokers’. The participants reported whether they had history of CAD or any family history of CAD. Finally, the participants reported if they live a sedentary lifestyle or were generally physically active.
Statistical analysis
Descriptive analyses were performed for demographics, MPA diameter, and CV risk factors. In addition, t-test was performed to assess mean MPA diameter by different CV risk factors. Unadjusted and adjusted linear regression analyses were performed to assess the associations between CV risk factors and MPA diameter. For significance, p value was set at 0.05 for two-tailed test. The data were analyzed using SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Overall, the age range for the total sample was 23 to 90 years with mean age of 58±10 years. The majority of the participants aged ≤60 years (59.7%). Slightly more than half of the participants were females (51.3%). Around 15.0% of them have been diagnosed with diabetes or were using medication to control blood sugar level. In addition, 49.5% and 55.3% of the participants had hypertension and hypercholesterolemia, respectively. Slightly more than one-third of the participants (35.8%) had ever smoked a cigarette. While 66.4% of the participants had family history of CAD, only 28.5% had a personal history of CAD. Additionally, around 40% of the participants reported that they had a sedentary lifestyle. The mean BMI of the study population was found to be 29.37±5.87 kg/m2 and 39.6% were obese. While the mean MPA diameter was 26.06±4.19 mm that of ascending aorta was 33.78±4.40. The correlation coefficient between diameter of MPA and ascending aorta was 0.09314.
The mean MPA diameter was significantly higher among males compared to females (27.19±4.20 vs. 24.99±3.91 mm, P<0.0001). Study established the MPA diameter as 29 mm in men and 27 mm in women as sex-specific normative reference in Framingham Heart Study (FHS). Participants of this study have lower mean MPA diameter in both males and females as compared to FHS (29). This reference was used because both Framingham Heart Study and our study population are predominantly white and asymptomatic as well. Participants with diabetes also had wider MPA diameter (26.79±4.56 mm) compared to those without diabetes (25.93±4.11 mm) (P=0.015). Further, hypertensive (26.42±4.15 vs. 25.71±4.21 mm, P=0.002) and obese (27.25±4.11 vs. 25.28±4.07 mm, P<0.0001) participants had significantly wider MPA compared to non-hypertensive and non-obese participants respectively. The mean MPA diameter did not significantly differ by age category, hypercholesterolemia, smoking status, family history of CAD, personal history of CAD or sedentary lifestyle (Table 1).
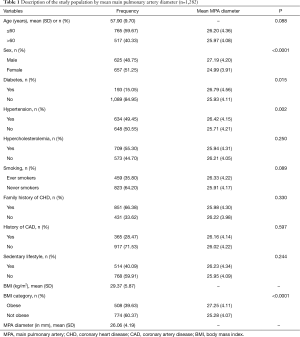
Full table
In unadjusted regression model, sex, diabetes, hypertension, and BMI were found to be significantly associated with increased MPA diameter. Participants with diabetes and hypertension were found to have 0.86 mm (P=0.008) and 0.71mm (P=0.002) increases in MPA diameter, as compared to non-diabetic and non-hypertensive respectively. However, in adjusted model, only age, sex and BMI were found to be significantly associated with increased MPA diameter. Thus, an increase in age by one year was associated with increased MPA diameter by 0.046 mm (P<0.0001). The diameter of MPA was wider among males by 2.16 mm, compared to females (P<0.0001). Finally, a one unit increase in BMI increased the MPA diameter by 0.16 mm (P<0.0001; Table 2).

Full table
Discussion
There has been an increasing trend to screen for at risk patients for PH and patients with other pulmonary diseases who present with symptoms such as shortness of breath and fatigue with non-invasive modalities to prevent long-term morbidity and mortality. Introduction of non-invasive imaging techniques to measure the diameter of the MPA led to advances in the research linking the diameter of the MPA to cardiopulmonary risk factors. Studies done in this area have found that the diameter of MPA to be significantly associated with age, sex and body surface area (11-18). In our study, we measured the MPA diameter in asymptomatic population with multiple CV risk factors using CT scan to determine the association between these risk factors and changes in the diameter.
Unadjusted regression analysis results suggest that MPA diameter is associated with sex, diabetes, hypertension and BMI. It was found that having diabetes or hypertension increases the MPA diameter by 0.86 and 0.71 mm, as compared to non-diabetic and non-hypertensive respectively. However, in the adjusted model, only age, sex and BMI were significantly associated with MPA. These findings corroborate with the findings in study done by Lee et al. showing that male sex and obesity, were associated with a larger MPA (12).
We found a significant association between the MPA diameter and sex; the diameter of MPA was wider among males by 2.16 mm as compared to females, which is consistent with the findings of most of the studies that observed significant differences in MPA diameter between males and females (13-21,24-30,35,36). Age was significantly associated with the MPA diameter, and this finding also corroborates with results of a study where the diameter of the MPA increased with age (31). However, another study found no statistically significant associations between age and MPA diameter (17). BMI is significantly associated with the MPA, with one-unit increase in BMI being associated with a 0.16 mm increase in the MPA diameter. In the literature, this association was studied using body surface area; which was significantly associated with the MPA (29); no study was found linking the BMI with the MPA diameter. Although in some studies, the MPA diameter measured by CT scan revealed association with the presence of PH, whether the strength of correlation has any significant implication in clinical practice to assess for PH is still unknown. It is also uncertain whether non-invasive techniques, including CT or MRI, have any implication on diagnosing or excluding the PH better than readily available echocardiogram. A cohort study done by Chan et al. (37). Of the101 patients with cardio-pulmonary conditions who had a CT chest and right heart catheterization performed three days apart revealed that the MPA was a poor predictor of PH. However, after adjusting for age, sex, thoracic diameter, ascending aortic diameter and pulmonary wedge pressure, the accuracy of the model improved significantly with area under the curve of 0.93. This suggests that there are multiple factors affecting the MPA diameter and PH (37).
This study has potential sources of error, which is primarily how the MPA was ascertained. These sources of error include differences in age, sex, body surface area, image slice thickness, CT window width and level, difficulties in identifying vessel interfaces, use of intravenous contrast (may transiently affect the PA diameter by affecting vascular tone, cardiac output, and/or heart rate), inclusion of arterial wall in the measurements (leading edge to leading edge or intima to adventitia), and period of the cardiac cycle (systole or diastole) when images are obtained (11,21). Nevertheless, standard protocol was used to ascertain the MPA (34). Moreover, this is likely the foremost study of the association between CV risk factors and MPA diameter among residents in Central Appalachia. Added to that is the large sample size, which makes the outcome of the study more reliable compared to previous studies. Another strength of the study is that we have almost equal representation of males and females and we excluded any outlier from the analysis.
Conclusions
MPA diameter was significantly associated with age, sex, and BMI. Further prospective studies are needed to correlate CT measurement of MPA diameter with pulmonary pressure as assessed by echocardiogram to diagnose PH as more imaging studies are done in patients with several lung conditions.
Acknowledgments
The researchers would like to thank the Department of Health Services Management and Policy, College of Public Health, at East Tennessee State University (ETSU) for the logistical support. We would also like to thank Legacy Wellmont Health System (now Ballad Health), for the collaboration and for providing the researchers with access to data. Further, we would like to thank the Office of Diversity and Equity at East Tennessee State University for providing the funding that supported research assistants who painstakingly collected the data for this paper.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the Collaborating Health System and the authors’ institution and written informed consent was obtained from all patients.
References
- Patel DJ, De Freitas FM, Mallos AJ. Mechanical function of the main pulmonary artery. J Appl Physiol 1962;17:205-8. [Crossref] [PubMed]
- Reuben SR. Compliance of the Human Pulmonary Arterial System in Disease. Circ Res 1971;29:40-50. [Crossref] [PubMed]
- Hopkins RA, Hammon JW Jr, McHale PA, et al. An Analysis of the Pulsatile Hemodynamic Responses of the Pulmonary Circulation to Acute and Chronic Pulmonary Venous Hypertension in the Awake Dog. Circ Res 1980;47:902-10. [Crossref] [PubMed]
- Zuckerman BD, Orton EC, Stenmark KR, et al. Alteration of the pulsatile load in the high-altitude calf model of pulmonary hypertension. J Appl Physiol 1991;70:859-68. [Crossref] [PubMed]
- Milnor WR, Jose AD, Mcgaff CJ. Pulmonary Vascular Volume, Resistance, and Compliance in Man. Circulation 1960;22:130-7. [Crossref] [PubMed]
- Greenfield JC, Griggs DM. Relation between pressure and diameter in main pulmonary artery of man. J Appl Physiol 1963;18:557-9. [Crossref] [PubMed]
- Milnor WR, Conti CR, Lewis KB, et al. Pulmonary Arterial Pulse Wave Velocity and Impedance in Man. Circ Res 1969;25:637-49. [Crossref] [PubMed]
- Sanz J, Kariisa M, Dellegrottaglie S, et al. Evaluation of Pulmonary Artery Stiffness in Pulmonary Hypertension With Cardiac Magnetic Resonance. JACC Cardiovasc Imaging 2009;2:286-95. [Crossref] [PubMed]
- O’Rourke MF, Yaginuma T, Avolio AP. Physiological and pathophysiological implications of ventricular/vascular coupling. Ann Biomed Eng 1984;12:119-34. [Crossref] [PubMed]
- Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther 2001;92:1-20. [Crossref] [PubMed]
- Mesquita SM, Castro CR, Ikari NM, et al. Likelihood of left main coronary artery compression based on pulmonary trunk diameter in patients with pulmonary hypertension. Am J Med 2004;116:369-74. [Crossref] [PubMed]
- Lee SH, Kim YJ, Lee HJ, et al. Comparison of CT-Determined Pulmonary Artery Diameter, Aortic Diameter, and Their Ratio in Healthy and Diverse Clinical Conditions. PLoS One 2015;10:e0126646. [Crossref] [PubMed]
- Lange TJ, Bornia C, Stiefel J, et al. Increased Pulmonary Artery Diameter on Chest Computed Tomography Can Predict Borderline Pulmonary Hypertension. Pulm Circ 2013;3:363-8. [Crossref] [PubMed]
- McCall RK, Ravenel JG, Nietert PJ, et al. Relationship of main pulmonary artery diameter to pulmonary arterial pressure in scleroderma patients with and without interstitial fibrosis. J Comput Assist Tomogr 2014;38:163-8. [Crossref] [PubMed]
- Tan RT, Kuzo R, Goodman LR, et al. Utility of CT Scan Evaluation for Predicting Pulmonary Hypertension in Patients With Parenchymal Lung Disease. Chest 1998;113:1250-6. [Crossref] [PubMed]
- Pérez-Enguix D, Morales P, Tomás JM, et al. Computed Tomographic Screening of Pulmonary Arterial Hypertension in Candidates for Lung Transplantation. Transplant Proc 2007;39:2405-8. [Crossref] [PubMed]
- Mahammedi A, Oshmyansky A, Hassoun PM, et al. Pulmonary Artery Measurements in Pulmonary Hypertension. J Thorac Imaging 2013;28:96-103. [Crossref] [PubMed]
- Devaraj A, Wells AU, Meister MG, et al. Detection of Pulmonary Hypertension with Multidetector CT and Echocardiography Alone and in Combination. Radiology 2010;254:609-16. [Crossref] [PubMed]
- Grubstein A, Benjaminov O, Dayan DB, et al. Computed tomography angiography in pulmonary hypertension. Isr Med Assoc J 2008;10:117-20. [PubMed]
- Kuriyama K, Gamsu G, Stern RG, et al. CT-determined pulmonary artery diameters in predicting pulmonary hypertension. Invest Radiol 1984;19:16-22. [Crossref] [PubMed]
- Moore NR, Scott JP, Flower CD, et al. The relationship between pulmonary artery pressure and pulmonary artery diameter in pulmonary hypertension. Clin Radiol 1988;39:486-9. [Crossref] [PubMed]
- Mohamed Hoesein FA, Besselink T, Pompe E, et al. Accuracy of CT Pulmonary Artery Diameter for Pulmonary Hypertension in End-Stage COPD. Lung 2016;194:813-9. [Crossref] [PubMed]
- Thenappan T, Prins KW, Pritzker MR, et al. The Critical Role of Pulmonary Arterial Compliance in Pulmonary Hypertension. Ann Am Thorac Soc 2016;13:276-84. [PubMed]
- Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and Diagnosis of Pulmonary Hypertension. J Am Coll Cardiol 2013;62:D42-50. [Crossref] [PubMed]
- Brown LM, Chen H, Halpern S, et al. Delay in Recognition of Pulmonary Arterial Hypertension: Factors Identified From the REVEAL Registry. Chest 2011;140:19-26. [Crossref] [PubMed]
- Rich S, Dantzker DR, Ayres SM, et al. Primary Pulmonary Hypertension. Ann Intern Med 1987;107:216. [Crossref] [PubMed]
- Nguyen ET, Silva CIS, Seely JM, et al. Pulmonary Artery Aneurysms and Pseudoaneurysms in Adults: Findings at CT and Radiography. AJR Am J Roentgenol 2007;188:W126-34. [Crossref] [PubMed]
- Raymond TE, Khabbaza JE, Yadav R, et al. Significance of Main Pulmonary Artery Dilation on Imaging Studies. Ann Am Thorac Soc 2014;11:1623-32. [Crossref] [PubMed]
- Truong QA, Massaro JM, Rogers IS, et al. Reference values for normal pulmonary artery dimensions by noncontrast cardiac computed tomography: the Framingham Heart Study. Circ Cardiovasc Imaging 2012;5:147-54. [Crossref] [PubMed]
- Nevsky G, Jacobs JE, Lim RP, et al. Sex-Specific Normalized Reference Values of Heart and Great Vessel Dimensions in Cardiac CT Angiography. AJR Am J Roentgenol 2011;196:788-94. [Crossref] [PubMed]
- Edwards PD, Bull RK, Coulden R. CT measurement of main pulmonary artery diameter. Br J Radiol 1998;71:1018-20. [Crossref] [PubMed]
- Pellegrini P, Rossi A, Pasotti M, et al. Prognostic Relevance of Pulmonary Arterial Compliance in Patients With Chronic Heart Failure. Chest 2014;145:1064-70. [Crossref] [PubMed]
- Al-Naamani N, Preston IR, Paulus JK, et al. Pulmonary Arterial Capacitance Is an Important Predictor of Mortality in Heart Failure With a Preserved Ejection Fraction. JACC Heart Fail 2015;3:467-74. [Crossref] [PubMed]
- Freeman LA, Young PM, Foley TA, et al. CT and MRI Assessment of the Aortic Root and Ascending Aorta. AJR Am J Roentgenol 2013;200:W581-92. [Crossref] [PubMed]
- Guthaner DF, Wexler L, Harell G. CT demonstration of cardiac structures. AJR Am J Roentgenol 1979;133:75-81. [Crossref] [PubMed]
- Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary Arterial Hypertension: Baseline Characteristics From the REVEAL Registry. Chest 2010;137:376-87. [Crossref] [PubMed]
- Chan AL, Juarez MM, Shelton DK, et al. Novel computed tomographic chest metrics to detect pulmonary hypertension. BMC Med Imaging 2011;11:7. [Crossref] [PubMed]


