
PEEP titration guided by transpulmonary pressure: lessons from a negative trial
Background
Since the first description of the acute respiratory distress syndrome (ARDS) by the landmark paper of Ashbaugh et al. (1), the adequate use of positive end-expiratory pressure (PEEP) has been surrounded by a vivid controversy. This stems from the fact that its beneficial effects on oxygenation by re-aerating collapsed or flooded airspaces, may be counterbalanced by potential adverse effects on hemodynamics and on the risk of increasing lung tissue mechanical stress. The vast amount of clinical and experimental reports over the last five decades, adequately reflect this “PEEP paradox”: the simultaneous effects of PEEP on gas exchange, lung mechanics and hemodynamics can have competing beneficial or deleterious consequences even in similar clinical or experimental conditions. Thus, the effects of PEEP are complex and difficult to predict, more so in the heterogeneous ARDS lung, and depend not only on the selected level but also on how this level interacts and modifies the lung status. For instance, a high PEEP level may improve oxygenation but if it not associated to significant recruitment of collapsed lung regions can increase non-dependent lung overdistension.
Although in clinical practice the changes in oxygenation remain the main driver for PEEP selection, the progressive awareness that mechanical ventilation can aggravate lung injury has shifted the interest to the potential lung-protective effects of PEEP, already recognized in early experimental studies (2). By preventing end-expiratory lung collapse and increasing end-expiratory lung volume, PEEP can counteract the two major mechanisms related to ventilation-induced lung injury (VILI) (3). On the one hand, it reduces or avoids the strain resulting from cyclic recruitment-derecruitment in boundary-regions of the mid-dependent regions, between collapsed and aerated lung. On the other hand, it promotes a more homogeneous distribution of ventilation by increasing the size of the functional lung thereby reducing the cyclic inflation stress of the non-dependent lung. Lung-protective ventilation (LPV) strategies aimed at reducing the mechanical stress on the lung, are the only therapeutic interventions that have improved ARDS outcome, and although the ultimate contribution of VILI to mortality is not known, it is important to emphasize that only a fifth of ARDS patients die with refractory hypoxemia (4). However, the definitive role of PEEP in lung protection has been difficult to establish in clinical studies (5). Dichotomous high-vs-low PEEP study designs, failure to confirm patient responsiveness to PEEP (6), and absence of proper PEEP individualization are among the reasons behind this lacking evidence.
PEEP in the era of LPV strategies
Currently, the clinical selection of PEEP during LPV strategies can be narrowed down to two main conceptual approaches: the ARDSnet approach (7) focused on oxygenation, and the open lung approach (OLA) (8) focused on lung protection. The ARDSnet PEEP-strategy is based on the concept of the minimum PEEP level necessary to reach a conservative oxygenation target. For this purpose, an empirically constructed PEEP/inspired oxygen fraction (FIO2) table, based on expert opinion is used. The table allows to choose between several PEEP levels depending on the FIO2 requirements. Although never specifically tested in a randomized controlled trial, the PEEP/FIO2 table is considered the standard of care and used as reference in most comparative studies on LPV strategies. Advocates of this pragmatic method argue that it is relatively easy to use in uncomplicated ARDS patients in which PEEP is usually selected from mid-table values, resulting in levels between 8–12 cmH2O. However, its lack of pathophysiological basis becomes evident in more complex patients with heterogeneous lungs where the table can become difficult to use. For instance, when oxygenation improves in response to PEEP and FIO2 can be decreased to less-toxic levels, the table forces one to reduce PEEP, eventually losing its clinical benefits.
Even more problematic is the patient not responding to PEEP. In a patient who remains hypoxemic, the table advises to further increase PEEP seeking an improvement in oxygenation. This may lead to dangerous unnecessary increases in transpulmonary and mean airway pressures, augmenting lung tissue stress and impairing hemodynamics, without any clinical benefit. The inherent problem of the PEEP/FIO2 table is that it is based solely on oxygenation criteria which may be misleading when lung protection is the principal concern. Furthermore, changes in oxygenation are influenced by many factors such as ventilation-perfusion inequalities, alterations in pulmonary perfusion, mixed venous oxygenation and hypoxic pulmonary vasoconstriction, that can be completely detached from changes in lung mechanics or lung tissue stress. With variability between countries, the clinical adoption of the PEEP/FIO2 table seems however, to be rather limited as suggested by large observational studies (9).
When using an OLA strategy, lung protection is the primary concern for PEEP selection. The rationale here is that the combination of a brief recruitment maneuver followed by an individualized PEEP selection can maintain the lung open. The PEEP titration is performed by means of a decremental PEEP trial during which PEEP levels are stepwise decreased searching for the minimum level that prevents lung de-recruitment (i.e., the closing pressure). This level can be detected by following the changes in several physiologic parameters such as respiratory system compliance (8,10), oxygenation (11), functional imaging techniques such as electrical impedance tomography (12) and more recently end-expiratory transpulmonary pressure (PTP) (13,14). Ideally, in a responding patient the final PEEP level results in the best compromise between non-dependent lung overdistension and dependent lung collapse. Of note, two premises need to be met when using this method. First, lung collapse must be an important component in the pathophysiology of the respiratory failure. Second, the lung has to be recruitable. For this latter condition, it is essential to individualize the recruitment maneuver, applying effective opening pressures in each individual patient when assessing recruitability (15,16). In non-responding patients this method cannot be applied effectively and PEEP levels must be kept at moderate levels. Potential alternatives to guide PEEP selection could then include methods such as the stress-index, a method based on the analysis of the shape of the pressure time curve during constant flow inflation aimed at minimizing cyclic overdistension (17). In responding patients, the identification of the adequate PEEP level is critical. As PEEP levels are generally higher than those obtained from the PEEP/FIO2 table, it is essential to appropriately monitor changes in the chosen lung physiologic parameters that define the closing pressure during the decremental PEEP trial to avoid PEEP over-or-underestimations. An overestimation will increase the risk of the cyclic stress in non-dependent alveoli, as a minimum inspiratory driving pressure will be limited by alveolar ventilation.
PEEP selection guided by PTP
A new method to titrate a lung-protective level of PEEP based on the measurement of PTP has been recently proposed (18). This method is especially appealing as PTP directly reflects alveolar distending pressure without the influence of the pressure needed to expand the chest wall, and thus is intimately related to the cyclic stress of lung tissue. PTP is the difference between airway and pleural pressure (Ppl), the pressure surrounding the alveoli. As Ppl cannot be directly measured, esophageal manometry using an esophageal catheter with an air-filled balloon at its tip can be used to measure the esophageal pressure (Pes) as a surrogate. Absolute PTP values can thus be measured clinically at end-inspiration (PTP-EI = Plateau pressure – Pes at end inspiration) or at end-expiration (PTP-EE = PEEP – Pes at end-expiration). Although its validity has been questioned (19), it is now recognized that when correctly placed, Pes provides reliable absolute estimates of Ppl at the iso-gravitational horizontal plane at which the balloon is positioned (20), generally corresponding to the mid-dependent portion of the lung, the one most relevant to lung collapse and thus for PEEP titration. In normal physiological conditions PTP remains positive keeping alveoli expanded at end-expiration when PTP-EE is about 1 to 2 cmH2O (21). Due to the vertical gravitational gradient of Ppl, PTP is also affected by regional variations, being more positive in non-dependent than in dependent lung areas where Ppl is higher. These regional differences are greatly amplified during mechanical ventilation in the supine position especially in the heterogenous-edematous ARDS lung in which Ppl reaches higher positive values in the dependent lung so that PTP-EE can become negative if the applied PEEP is below Ppl, promoting lung collapse.
The principle of applying an Pes guided method for PEEP titration was successfully tested in the esophageal pressure-guided ventilation (EPVent) trial (18). In this single center pilot study including a small patient population, an Pes-guided PEEP titration method was compared with the tabular PEEP/FIO2 method (7). The Pes-guided PEEP method selected a level to obtain a polarity change of PTP-EE from negative, associated to dependent lung collapse, to positive values (22) at a level similar or above the measured Pes. How much the PEEP was allowed to divert from Pes, or in other words, how positive PTP-EE was targeted, was determined by the need of FIO2 in a similar way as the PEEP/FIO2 table. For this purpose, an empirical PTP-EE/FIO2 table was applied allowing PTP-EE values from 0, for the lowest FIO2 needs, to +6 cmH2O for the highest needs. With an average PEEP difference of 6 cmH2O between both groups during the first 72 h after enrollment, the Pes-guided method resulted in significantly better oxygenation (42% increase in PaO2/FIO2) and lung mechanics (45% better compliance) and in a trend toward a lower mortality (17% vs. 39%, P=0.055). The study was stopped early once the primary endpoint criterion (improvement in oxygenation) was reached.
The EPVent-2 trial, a missed opportunity
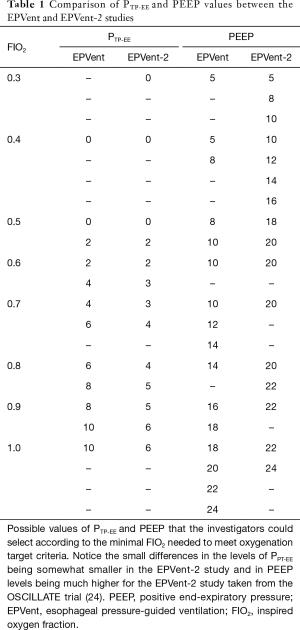
The encouraging outcomes reported in the EPVent study generated a great expectation and triggered the recently published EPVent-2 trial which unfortunately did not confirm these promising results (23). The EPVent-2 study, a phase II multicenter randomized controlled trial compared a similar Pes-guided PEEP titration strategy as the EPVent trial (with slightly decreased values, Table 1) with a modified PEEP/FIO2-table using higher PEEP values (24) in moderate-severe ARDS patients submitted to LPV. They included 200 patients from 14 academic hospitals having a composite hierarchically outcome of mortality and ventilator-free days at 28 days as primary endpoint. Each patient-patient comparison received a score that ranked worse for mortality than for days off the ventilator. The effect size was then expressed with a probabilistic index, the estimated probability that an individual randomly assigned to one group would have a more favorable outcome (a higher score) than one assigned to the other group. This probability was 49.6% [95% confidence interval (CI), 41.7% to 57.5%] for the Pes-guided group and 50.4% (95% CI, 42.5% to 58.3%) in the control group. All other secondary outcomes were similar, excepting the need for rescue therapies (3.9% vs. 12.2%) in favor of the Pes-guided group. Surprisingly, none of the physiological variables of oxygenation and PTP were different between groups.
Full table
There are several reasons that could explain these unexpected negative results that on the one hand failed to reproduce the physiological benefits of the previous EPVent trial and on the other hand did not improve outcome despite using a ranked composite endpoint, in theory increasing the chances for obtaining a positive effect (25). First, there were important differences with the EPVent trial. The EPVent-2 was a multicenter study with variable levels of expertise in the different centers in implementing a novel and clinically challenging method as opposed to the high expertise single center EPVent study. Patient populations were also different: EPVent had fewer severe ARDS and a higher proportion of surgical patients. Oxygenation was the primary endpoint for the EPVent study which led to an early stop for benefit, risking an overestimation of the true effect, whereas the EPVent-2 had a patient-centered primary endpoint and was completed with all planned patients enrolled. The EPVent-2 used a modified PEEP/FIO2-table using significantly higher levels of PEEP (Table 1). This resulted in similar average values of PEEP, PTP-EI, PTP-EE, plateau and driving pressure in both groups during the first seven days. Although those findings do not preclude the existence of individual differences, they indicate that in practice both treatments were similar.
Second, influenced by the positive EPVent-1 results, investigators chose a rather optimistic estimation of the effect size. A 22% absolute difference in mortality was a “hard to believe” finding in a trial with an oxygenation target, especially when the causes of death were not reported.
Third, a more fundamental critique is related to the limitations of the PEEP titration method per se. Although the proposed PTP-EE polarity change method makes pathophysiological sense in terms of lung protection, linking the final level to the oxygenation criteria by an arbitrarily constructed table, was an erroneous decision. We have already discussed the limitations of using a PEEP/FIO2 table when lung protection is the primary concern and how oxygenation criteria do not necessarily parallel changes in lung mechanics. A patient with limited oxygenation would have been at risk for an excessive PEEP and consequently submitted to a higher mechanical stress. This was in part compensated by the higher PEEP/FIO2 table used in the control group in which for example, a patient requiring a FIO2 ≥0.5 was managed with a PEEP ≥18 cmH2O, a level most likely excessive for some patients. Furthermore, PTP-EE, calculated at the level at which the esophageal balloon is placed did not guarantee that alveoli located at most dependent regions maintained a positive value, and thus remained collapsed. Although the investigators are known experts and Pes waveform recordings were analyzed and checked for quality-control in the core laboratory, Pes estimation is not easy, and prone to measurement artifacts. Reliable measurements depend on proper positioning and adequate inflation of the balloon and errors in estimating PEEP, especially at higher values, can rapidly shift the balance toward detrimental effects. Finally, the fact that both groups were monitored with an esophageal catheter may have introduced an unwanted bias.
Future perspectives
Despite the negative results of the EPVent-2 trial, the physiological meaning of PTP can be very useful in the individual management of ARDS patients. Its measurement may be particularly convenient in situations with increased chest-wall stiffness and high Ppl such as in morbidly obese patients or situations of abdominal hypertension were large differences between airway pressure and regional PTP are expected (26). In such circumstances, Pes and PTP could help in the appropriate selection of “safe” airway pressure levels even if above the clinical “comfort zone”. However, considering that chest-wall mechanics is usually minimally affected in ARDS patients and that the differences observed between airway and transpulmonary driving pressures in the EPVent-2 study were minimal, it will be important to determine whether monitoring PTP with its laborious clinical application offers clear advantages in patients without excessive increases in Ppl or those with normal chest wall mechanics. Regarding the selection of PEEP, the PTP-EE method shares similar lung-protective targets as the decremental PEEP trial method, namely minimizing dependent lung collapse. Comparative studies have shown that optimum PEEP levels are similar with both methods (27) and that a PTP-EE of around 2 cmH2O corresponds to the closing pressure detected during a decremental PEEP trial (13). However, to be comparable, the PTP-EE method must be used in combination with a previous lung recruitment and in such a context the question is whether PTP-EE offers any measurable advantage over other physiological variables measured during a decremental PEEP trial, such as lung compliance.
In summary, the quest for identifying a method for optimizing PEEP in ARDS patients continues. Initiatives such as the introduction of PTP to aid in the selection of the correct level of PEEP are welcome as they provide additional information that can be useful in the individual patient to assess the physiological response and contribute to improve lung protection.
Acknowledgments
Funding: Partially supported by the Instituto de Salud Carlos III (PI16/00049, PI18/01611) and by the European Funds for Regional Development.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Ashbaugh DG, Bigelow DB, Petty TL, et al. Acute respiratory distress in adults. Lancet 1967;2:319-23. [Crossref] [PubMed]
- Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 1974;110:556-65. [PubMed]
- Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med 2013;369:2126-36. [Crossref] [PubMed]
- Villar J, Martínez D, Mosteiro F, et al. Is overall mortality the right composite endpoint in clinical trials of acute respiratory distress syndrome? Crit Care Med 2018;46:892-9. [Crossref] [PubMed]
- Walkey AJ, Del Sorbo L, Hodgson CL, et al. Higher PEEP versus lower PEEP strategies for patients with acute respiratory distress syndrome. A systematic review and meta-analysis. Ann Am Thorac Soc 2017;14:S297-S303. [Crossref] [PubMed]
- Goligher EC, Kavanagh BP, Rubenfeld GD, et al. Physiologic responsiveness should guide entry into randomized controlled trials. Am J Respir Crit Care Med 2015;192:1416-9. [Crossref] [PubMed]
- Acute Respiratory Distress Syndrome Network, Brower RG, Matthay MA, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000;342:1301-8. [Crossref] [PubMed]
- Kacmarek RM, Villar J, Sulemanji D, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med 2016;44:32-42. [Crossref] [PubMed]
- Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016;315:788-800. [Crossref] [PubMed]
- Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial (ART) Investigators, Cavalcanti AB, Suzumura ÉA, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2017;318:1335-45. [Crossref] [PubMed]
- Hodgson CL, Tuxen DV, Davies AR, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care 2011;15:R133. [Crossref] [PubMed]
- Costa EL, Borges JB, Melo A, et al. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 2009;35:1132-7. [Crossref] [PubMed]
- Fumagalli J, Berra L, Zhang C, et al. Transpulmonary pressure describes lung morphology during decremental positive end-expiratory pressure trials in obesity. Crit Care Med 2017;45:1374-81. [Crossref] [PubMed]
- Ferrando C, Tusman G, Suarez-Sipmann F, et al. Individualized lung recruitment maneuver guided by pulse-oximetry in anesthetized patients undergoing laparoscopy: a feasibility study. Acta Anaesthesiol Scand 2018;62:608-19. [Crossref] [PubMed]
- Borges JB, Okamoto VN, Matos GF, et al. Reversibility of lung collapse and hypoxemia in early acute respiratory distress syndrome. Am J Respir Crit Care Med 2006;174:268-78. [Crossref] [PubMed]
- de Matos GF, Stanzani F, Passos RH, et al. How large is the lung recruitability in early acute respiratory distress syndrome: a prospective case series of patients monitored by computed tomography. Crit Care 2012;16:R4. [Crossref] [PubMed]
- Grasso S, Stripoli T, De Michele M, et al. ARDSnet ventilatory protocol and alveolar hyperinflation: role of positive end-expiratory pressure. Am J Respir Crit Care Med 2007;176:761-7. [Crossref] [PubMed]
- Talmor D, Sarge T, Malhotra A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 2008;359:2095-104. [Crossref] [PubMed]
- Loring SH, O'Donnell CR, Behazin N, et al. Esophageal pressures in acute lung injury: do they represent artifact or useful information about transpulmonary pressure, chest wall mechanics, and lung stress? J Appl Physiol 1985;2010:515-22. [PubMed]
- Yoshida T, Amato MBP, Grieco DL, et al. Esophageal manometry and regional transpulmonary pressure in lung injury. Am J Respir Crit Care Med 2018;197:1018-26. [Crossref] [PubMed]
- Faffe DS, Zin WA. Lung parenchymal mechanics in health and disease. Physiol Rev 2009;89:759-75. [Crossref] [PubMed]
- Marini JJ. Should we titrate positive end-expiratory pressure based on an end-expiratory transpulmonary pressure? Ann Transl Med 2018;6:391. [Crossref] [PubMed]
- Beitler JR, Sarge T, Banner-Goodspeed VM, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA 2019;321:846-57. [Crossref] [PubMed]
- Ferguson ND, Cook DJ, Guyatt GH, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med 2013;368:795-805. [Crossref] [PubMed]
- Finkelstein DM, Schoenfeld DA. Combining mortality and longitudinal measures in clinical trials. Stat Med 1999;18:1341-54. [Crossref] [PubMed]
- Pelosi P, Luecke T, Rocco PR. Chest wall mechanics and abdominal pressure during general anaesthesia in normal and obese individuals and in acute lung injury. Curr Opin Crit Care 2011;17:72-9. [Crossref] [PubMed]
- Pirrone M, Fisher D, Chipman D, et al. Recruitment maneuvers and positive end-expiratory pressure titration in morbidly obese ICU patients. Crit Care Med 2016;44:300-7. [Crossref] [PubMed]


