
Outcomes and risk factors of postoperative hepatic dysfunction in patients undergoing acute type A aortic dissection surgery
Introduction
Acute aortic dissection is the most common medical emergency involving the aorta. The mortality of acute type A aortic dissection (AAAD) ranges from 40% to 50% within 48 hours of onset. Surgery for AAAD remains a challenge for cardiovascular surgeons (1-3). Hepatic dysfunction (HD) has been suggested as an important postoperative risk factors for mortality and morbidity after cardiovascular surgery (4,5). Moreover, due to the complexity of surgery, long operation time, and substantial trauma, AAAD is easily complicated by renal insufficiency, HD, and bowel ischemia after surgery, which leads to increased mortality (1). Studies focusing on HD after AAAD surgery are rare, and little is known about postoperative HD outcomes and risk factors.
In the past 30 years, the principal indicator of HD has been the Child-Turcotte-Pugh (CTP) classification (6). However, as an important clinical method for estimating hepatic function, the model for end-stage liver disease (MELD) score has been reported to constitute a more useful assessment tool to predict postoperative mortality and morbidity than CTP classification in patients undergoing cardiac surgery (5,7). MELD scores have been verified as a measure of HD and are used as a tool to prioritize liver transplant candidates based on disease severity (8). Hence, we chose MELD scores as our tools to assess postoperative HD, based on the following clinical indices: (I) international normalized ratio (INR); (II) total serum bilirubin; (III) serum creatinine; and (IV) disease (bilious or alcoholic liver disease =0, all others =1). These score indicators are objective and easy to obtain (9). The aim of our study was to evaluate the risk factors of postoperative HD in patients with AAAD and its impact on clinical outcomes.
Methods
Patients
We retrospectively reviewed the medical records of patients who underwent surgery for AAAD at Changhai Hospital from April 2015 to April 2017. In most cases, enhanced computed tomography was used to diagnose AAAD and determine which vessels were affected by the dissection. This study was approved by the Committee on Ethics of Biomedicine Research, The Second Military Medical University, Shanghai (No. SMMUEC2018-012), with the need for individual patient consent waived.
Data collection
We collected data on patients’ age, sex, body mass index, comorbidities (e.g., hypertension, diabetes, chronic obstructive pulmonary disease), history of smoking and alcohol consumption, preoperative MELD score and renal insufficiency, and laboratory test results [creatinine, leukocytes, hemoglobin, alanine transaminase (ALT), aspartate transaminase (AST), albumin, γ-glutamyl transferase, total bilirubin, K+, Na+, lactic acid, PaO2, PaO2/FiO2 ratio].
We also collected data on time from symptom onset to surgery, preoperative shock, preoperative moderate/severe pericardial effusion, dissection involvement (celiac trunk, superior mesenteric artery, and renal artery), and intraoperative conditions [cardiopulmonary bypass (CPB) time, aortic cross clamp (ACC) time, deep hypothermic circulatory arrest, and surgical procedure]. Finally, we recorded the 24-h postoperative drainage volume, perioperative blood product transfusion, and postoperative laboratory test results including ALT, AST, albumin, white blood cells, and INR.
Postoperative complications, such as low cardiac output syndrome (LCOS); pneumonia; renal insufficiency; respiratory dysfunction; mechanical ventilation time; time in the intensive care unit (ICU); need for reintubation, continuous renal replacement therapy (CRRT), tracheostomy; sepsis; re-exploration for bleeding; and blood transfusion, including red blood cells, plasma, and cryoprecipitate, were also recorded.
Definitions and grouping
To calculate patients’ MELD scores, we used the following formula:
MELD = 11.2 In (INR) + 0.378 In (total bilirubin) + 0.957 In (creatinine) + 0.643 (cause)
where cause equaled 0 for bilious or alcoholic liver disease and 1 otherwise.
We used the results of laboratory tests within 7 days to calculate MELD score every day after surgery using the above formula, and the highest value of MELD was used to determine whether patients experienced early postoperative HD to avoid the influence of other factors such as infection. Considering that a few patients were taking warfarin during the study, which may have affected the INR and MELD scores, we collected the INR values of these patients before they took their medicine. We grouped patients based on their postoperative score into high-MELD (MELD ≥14) and low-MELD (MELD <14) groups. Some previous studies set a cutoff point of 13 for patients with cirrhosis undergoing cardiac surgery (10,11), and recent research predicted postoperative mortality and morbidity after cardiac surgery based on a cutoff of 12 (5). Moreover, a recent study on outcomes of patients undergoing cardiac surgery after liver transplantation showed that the optimal cutoff point for predicting late mortality is 13.8 (12). In another study, the Youden index identified the optimal MELD score cutoff value of 13.5 for predicting surgical mortality of cardiac surgery in liver transplant recipients (13). Consequently, considering that AAAD surgery is more complicated than other cardiac surgeries, and based on the optimal cutoff values of MELD scores for risk of mortality in previous studies, we used 14 as our cutoff point.
Renal insufficiency was defined by an estimated glomerular filtration rate of <60 mL/min/1.73 m2, and hyperbilirubinemia was defined by total bilirubin >3 mg/dL. Respiratory dysfunction was defined as inadequate oxygenation (PaO2 <60 mmHg with a FiO2 of 0.5 or PaO2/FiO2 ≤120) or ventilation (PCO2 >50 mmHg) during mechanical ventilatory support (14). LCOS was diagnosed if the patient required two or more inotropic medications to maintain systolic blood pressure above 90 mmHg and a cardiac output greater than 2.2 L/min/m2 postoperatively after adjusting the preload and correcting for all electrolyte or blood gas abnormalities (15). Transfusion threshold and policies were as follows: if the patient’s hematocrit was lower than 25, we transfused packed red cell to increase the intravascular volume and maintain satisfactory tissue oxygenation; if the patient was bleeding in the early postoperative period, blood component therapy, selected based upon identification of specific coagulation abnormalities by point-of-care testing and treatment algorithms, plasma infusion, or cryoprecipitate were considered: fresh frozen plasma was usually given at 2–4 units for average adults, cryoprecipitate was given 1 unit at 1 unit/7–10 kg of body weight. Massive transfusion was defined as ≥10 units of blood transfused (16). Prolonged ICU stay was defined as >7 days (17).
Statistical analysis
Continuous variables are presented as means ± standard deviation or medians and interquartile range. Comparisons of continuous variables between the two groups were performed using the Student’s t-test for variables with a normal distribution. The Wilcoxon rank-sum test was used for variables with a non-normal distribution. Categorical variables were analyzed using the Pearson’s chi-squared or Fisher’s exact test. Receiver operating characteristic (ROC) curve and the Youden index were used to determine the optimal cutoff value of MELD score for predicting the surgical risk of mortality.
Multivariate logistic regression analyses were performed to identify risk factors for a high MELD score. Significant variables (P<0.05) associated with a high MELD score in the univariate analysis and clinically relevant variables were included in the multivariate analysis. The multivariate regression analysis was performed using a forward stepwise (conditional) procedure to determine the independent significant prognostic factors. The multivariate regression analysis was performed to determine the effect of a postoperative high MELD score on mortality, prolonged ICU stay, and massive transfusion after adjusting for relevant confounding variables in the three models. All P-values were two-sided. Statistical significance was defined as P<0.05. All statistical analyses were performed using SPSS 24.0 (IBM Corp.).
Results
Of the 216 patients with AAAD who underwent surgery from April 2015 to April 2017, one patient was excluded due to persistent bleeding and death within 24 h after surgery; the remaining 215 patients were divided into high-MELD (MELD score ≥14; n=131) and low-MELD (MELD score <14; n=84) groups. Based on MELD scores, the incidence rate of early postoperative HD was 60.9%, with an in-hospital mortality rate of 16.8%.
Baseline and preoperative characteristics
Patients with high postoperative MELD scores tended to have high preoperative MELD scores and preoperative renal insufficiency. Laboratory examination showed that the high-MELD group had higher preoperative creatinine levels and leucocyte counts (Table 1).
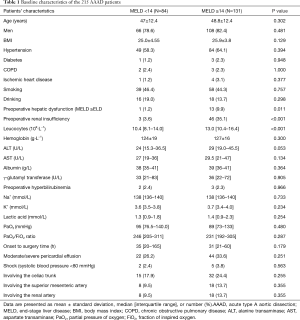
Full table
Surgery and postoperative characteristics
Patients in the high-MELD group had longer CPB and ACC times than those in the low-MELD group and underwent higher number of total-arch replacements and elephant trunk surgical procedures. Postoperative renal insufficiency occurred more frequently in the high-MELD group (72.5% vs. 20.2%). LCOS, respiratory dysfunction, and pneumonia were associated with high MELD scores. Patients with high MELD scores had a higher leucocyte count postoperatively and higher 24-h postoperative drainage volume (Table 2).

Full table
In-hospital outcomes of patients with high MELD scores
Patients with a high MELD score after surgery had a longer mechanical ventilation time, longer ICU stay, and higher in-hospital mortality rate. Patients with AAAD and a high MELD score needed more CRRT, reintubation, and tracheostomy after surgery. In addition, this group had a greater need for blood transfusion (Table 3).

Full table
Logistic regression for high postoperative MELD score
ACC time, postoperative leucocyte counts, postoperative LCOS, and postoperative respiratory dysfunction were independent predictive factors of a high postoperative MELD score (Table 4). After adjusting for relevant confounding variables, a high postoperative MELD score was associated with mortality and prolonged ICU stay, but not with massive transfusion (Tables 5,S1-S3).
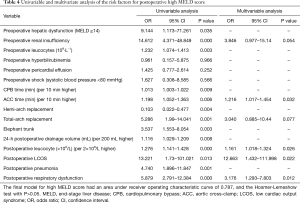
Full table
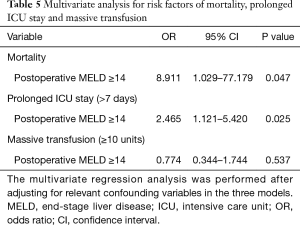
Full table

Full table

Full table

Full table
Discussion
The most important finding of our study was that a longer ACC time, higher postoperative leucocyte level, and postoperative LCOS and respiratory dysfunction were independent risk factors of postoperative HD in patients with AAAD.
Postoperative HD is a common serious complication in patients with AAAD. The MELD score has been used frequently in recent studies to evaluate hepatic function after cardiac surgery, but its use in AAAD surgery is relatively rare. Liu et al. reported that early postoperative HD incidence was 8.7% after AAAD surgery (18), while another study showed an HD incidence of 1.6% postoperatively among patients with AAAD, with a mortality rate of 38% (19). However, these studies relied on biochemical markers to evaluate hepatic function. In our study, we used the MELD score and found that the incidence of postoperative HD was as high as 60.9%, with an in-hospital mortality rate of 16.8%. We also found that highly suspected risk factors (e.g., peritoneal artery involvement, onset to surgery time) were not independent risk factors for postoperative HD.
Almost all open thoracic AAAD surgeries are performed with extracorporeal circulation i.e. CPB. Since CPB is a non-physiological state, and liver perfusion would be significantly reduced, leading to liver function damage. Although there is distal perfusion during the ACC time, liver perfusion is significantly reduced (20). It has previously been reported that the blood volume in liver arteries decreases by 20% to 25% during CPB (21). Hence, a long ACC time can induce a critical reduction in perfusion, and significant hypoxemia in the abdominal organs is inevitable, thus overwhelming the protective mechanisms and causing hypoxic liver damage (22). Furthermore, a long ACC time is associated with serious clinical conditions and complicated procedures can also lead to an inflammatory response. The subsequent activation of a variety of inflammatory mediators, such as adhesion molecules, thrombin, cytokines, endothelin and macrophages weakens the immune response, and a combination of these factors causes multi-organ dysfunction (23-25).
Increased postoperative HD was associated with a higher postoperative leucocyte count in our study. The leucocyte count is an important index for inflammatory response, and an early postoperative increase is highly associated with an inflammatory response rather than infection. When AAAD occurs, it activates multiple responses in the circulatory system, which can cause severe systemic inflammatory response syndrome and coagulation disorders. The inflammatory response is closely linked to the development of AAAD (26); however, due to its critical nature, rapid progression, requirement of a complex surgery, high requirements for extracorporeal circulation, and longer turnaround time, AAAD is more likely to cause a systemic inflammatory response. This is consistent with our previous discussion about ACC. In addition, CPB induces a certain degree of hemolysis, which promotes the release of free hemoglobin, production of endogenous substances play an important role in aggravating body’s inflammatory response (27), the risk of multi-organ failure increases, especially in the liver, kidney, lung, and other important organs.
As a common and serious complication in cardiac surgeries, postoperative respiratory dysfunction may prolong the ICU stay and increase in-hospital mortality (28,29). The incidence of postoperative hypoxemia is up to 51% after surgery for AAAD (30). In addition, prolonged respiratory dysfunction requires extended mechanical ventilation time, and the resulting hypoxia can damage vital organs, especially those that are more sensitive to hypoxia, such as the lung and liver. This damage causes other serious complications, such as sepsis and HD.
Pérez Vela et al. showed that patients who experienced LCOS after cardiac surgery had a poorer postoperative course, with a greater incidence of multi-organ failure and higher mortality (31). When LCOS develops, it directly causes insufficient hepatic perfusion pressure, leading to hepatic ischemia and hypoxia. In addition, inotropic agents used to improve patients’ cardiac output were found to increase myocardial consumption and in-hospital mortality (32), leading to ischemia of other organs. The treatment goal of LCOS is to promote tissue DO2 by providing appropriate hemodynamic support to avoid dysfunction and failure of critical organs (33), leading to a good clinical outcome.
This study has some limitations related to its retrospective design and the fact that all data was generated by a single center. The conclusions may be influenced by this center’s practice standards; thus, multi-center studies should be carried out to obtain further insights. Moreover, the MELD score includes INR in the formula, and thus, warfarin administration may have an impact on the score; perhaps the MELD-XI score that excludes INR may be more appropriate for such analysis (34), and we hope that research on this aspect will be carried out in future. Finally, a strength of this study was the use of the MELD score to estimate liver function. We set a score of 14 as the cutoff point, which was higher than that used in previous cardiac surgery studies. However, AAAD surgery is more complicated than other types of cardiac surgery, and we consider that a higher cutoff point is more reasonable in this setting. We look forward to further studies on the appropriate cutoff point for the MELD score to classify hepatic function in patients with AAAD.
Conclusions
Postoperative HD prolongs mechanical ventilation time and ICU stay, and it is associated with in-hospital mortality in patients with AAAD. A longer ACC time, higher postoperative leucocyte count, postoperative respiratory dysfunction, and postoperative LCOS are independent risk factors of HD in patients with AAAD undergoing surgery.
Acknowledgments
We want to thank the statistician of statistical guidance of this work: Weituo Zhang. PhD, worked in Clinical Research Center of Shanghai Jiao Tong University School of Medicine
Funding: This work was supported by the National Natural Science Foundation of China (No. 81873524) and a China Postdoctoral Science Foundation funded project (NO.2018M633709).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: This study was approved by the Committee on Ethics of Biomedicine Research, The Second Military Medical University, Shanghai (No. SMMUEC2018-012), with the need for individual patient consent waived. The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Pape LA, Awais M, Woznicki EM, et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J Am Coll Cardiol 2015;66:350-8. [Crossref] [PubMed]
- Wong DR, Lemaire SA, Coselli JS. Managing dissections of the thoracic aorta. Am Surg 2008;74:364-80. [PubMed]
- Hughes GC, Ganapathi AM, Keenan JE, et al. Thoracic endovascular aortic repair for chronic DeBakey IIIb aortic dissection. Ann Thorac Surg 2014;98:2092-7; discussion 2098. [Crossref] [PubMed]
- Araujo L, Dombrovskiy V, Kamran W, et al. The effect of preoperative liver dysfunction on cardiac surgery outcomes. J Cardiothorac Surg 2017;12:73. [Crossref] [PubMed]
- Murata M, Kato TS, Kuwaki K, et al. Preoperative hepatic dysfunction could predict postoperative mortality and morbidity in patients undergoing cardiac surgery: Utilization of the MELD scoring system. Int J Cardiol 2016;203:682-9. [Crossref] [PubMed]
- Ziser A, Plevak DJ, Wiesner RH, et al. Morbidity and mortality in cirrhotic patients undergoing anesthesia and surgery. Anesthesiology 1999;90:42-53. [Crossref] [PubMed]
- Dimarakis I, Grant S, Corless R, et al. Impact of hepatic cirrhosis on outcome in adult cardiac surgery. Thorac Cardiovasc Surg 2015;63:58-66. [PubMed]
- Freeman RB, Wiesner RH, Edwards E, et al. Results of the first year of the new liver allocation plan. Liver Transpl 2004;10:7-15. [Crossref] [PubMed]
- Kamath PS, Kim WR. Advanced Liver Disease Study G. The model for end-stage liver disease (MELD). Hepatology 2007;45:797-805. [Crossref] [PubMed]
- Morisaki A, Hosono M, Sasaki Y, et al. Risk factor analysis in patients with liver cirrhosis undergoing cardiovascular operations. Ann Thorac Surg 2010;89:811-7. [Crossref] [PubMed]
- Filsoufi F, Salzberg SP, Rahmanian PB, et al. Early and late outcome of cardiac surgery in patients with liver cirrhosis. Liver Transpl 2007;13:990-5. [Crossref] [PubMed]
- Harrington PB, McAlexander WW, Bryant AS, et al. Outcomes of Patients Who Undergo Cardiac Surgical Procedures After Liver Transplantation. Ann Thorac Surg 2017;103:541-5. [Crossref] [PubMed]
- Ota T, Rocha R, Wei LM, et al. Surgical outcomes after cardiac surgery in liver transplant recipients. J Thorac Cardiovasc Surg 2013;145:1072-6. [Crossref] [PubMed]
- Rady MY, Ryan T, Starr NJ. Early onset of acute pulmonary dysfunction after cardiovascular surgery: risk factors and clinical outcome. Crit Care Med 1997;25:1831-9. [Crossref] [PubMed]
- Maganti M, Badiwala M, Sheikh A, et al. Predictors of low cardiac output syndrome after isolated mitral valve surgery. J Thorac Cardiovasc Surg 2010;140:790-6. [Crossref] [PubMed]
- El-Menyar A, Abdelrahman H, Alhammoud A, et al. Prognostic Role of Shock Index in Traumatic Pelvic Fracture: A Retrospective Analysis. J Surg Res 2019;243:410-8. [Crossref] [PubMed]
- Atoui R, Ma F, Langlois Y, et al. Risk factors for prolonged stay in the intensive care unit and on the ward after cardiac surgery. J Card Surg 2008;23:99-106. [Crossref] [PubMed]
- Liu N, Sun LZ, Chang Q. Zhonghua Wai Ke Za Zhi 2010;48:1154-7. [The relative risk factors analysis of hepatic dysfunction following aortic dissection repair]. [PubMed]
- Achouh PE, Madsen K, Miller CC 3rd, et al. Gastrointestinal complications after descending thoracic and thoracoabdominal aortic repairs: a 14-year experience. J Vasc Surg 2006;44:442-6. [Crossref] [PubMed]
- Chacon MM, Schulte TE. Liver Dysfunction in Cardiac Surgery - What Causes It and Is There Anything We Can Do? J Cardiothorac Vasc Anesth 2018;32:1719-21. [Crossref] [PubMed]
- Mathie RT. Hepatic blood flow during cardiopulmonary bypass. Crit Care Med 1993;21:S72-6. [Crossref] [PubMed]
- Laribi S, Mebazaa A. Cardiohepatic syndrome: liver injury in decompensated heart failure. Curr Heart Fail Rep 2014;11:236-40. [Crossref] [PubMed]
- Sugano Y, Anzai T, Yoshikawa T, et al. Serum C-reactive protein elevation predicts poor clinical outcome in patients with distal type acute aortic dissection: association with the occurrence of oxygenation impairment. Int J Cardiol 2005;102:39-45. [Crossref] [PubMed]
- Shi S, Zhao Z, Liu X, et al. Perioperative risk factors for prolonged mechanical ventilation following cardiac surgery in neonates and young infants. Chest 2008;134:768-74. [Crossref] [PubMed]
- Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 3,269 CABG patients. Minerva Anestesiol 2007;73:615-21. [PubMed]
- Luo F, Zhou XL, Li JJ, et al. Inflammatory response is associated with aortic dissection. Ageing Res Rev 2009;8:31-5. [Crossref] [PubMed]
- Davis CL, Kausz AT, Zager RA, et al. Acute renal failure after cardiopulmonary bypass in related to decreased serum ferritin levels. J Am Soc Nephrol 1999;10:2396-402. [PubMed]
- Zhu G, Huang Y, Wei D, et al. Efficacy and safety of noninvasive ventilation in patients after cardiothoracic surgery: A PRISMA-compliant systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e4734. [Crossref] [PubMed]
- Nakajima T, Kawazoe K, Izumoto H, et al. Risk factors for hypoxemia after surgery for acute type A aortic dissection. Surg Today 2006;36:680-5. [Crossref] [PubMed]
- Canver CC, Chanda J. Intraoperative and postoperative risk factors for respiratory failure after coronary bypass. Ann Thorac Surg 2003;75:853-7; discussion 857-8. [Crossref] [PubMed]
- Pérez Vela JL, Jimenez Rivera JJ, Alcala Llorente MA, et al. Low cardiac output syndrome in the postoperative period of cardiac surgery. Profile, differences in clinical course and prognosis. The ESBAGA study. Med Intensiva 2018;42:159-67. [PubMed]
- Nielsen DV, Hansen MK, Johnsen SP, et al. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology 2014;120:1098-108. [Crossref] [PubMed]
- Lomivorotov VV, Efremov SM, Kirov MY, et al. Low-Cardiac-Output Syndrome After Cardiac Surgery. J Cardiothorac Vasc Anesth 2017;31:291-308. [Crossref] [PubMed]
- Wernly B, Lichtenauer M, Vellinga N, et al. Model for End-Stage Liver Disease Excluding INR (MELD-XI) score is associated with hemodynamic impairment and predicts mortality in critically ill patients. Eur J Intern Med 2018;51:80-4. [Crossref] [PubMed]


