
Blood transfusion associated lung injury
Introduction
Transfusions of blood and blood products are live-saving. However, transfusion associated lung-injury is a rare but life-threatening complication. Hypoxemia and pulmonary edema after transfusion of allogenic blood have been described as early as 1936 (1). Numerous case reports followed reporting of respiratory complications after transfusion that were attributed to pulmonary hypersensitivity (2), allergic pulmonary edema or pulmonary leukoagglutinin reactions (3). In the 1980s, Popovsky et al. were the first to investigate the syndrome systematically. They call it “transfusion-related acute lung injury (TRALI)” (4). Since then, research tries to shed light on the pathophysiology of lung injury after transfusion. However, many questions still remain unanswered. In this mini-review article, we introduce two cases of TRALI to discuss definition, epidemiology, pathophysiology, blood products, diagnosis, treatment and future directions in the field.
Case 1
A young healthy female pedestrian is hit by a car crossing the street. She is admitted with severe traumatic intracranial bleeding, ligamentous injury to the cervical spine and chest contusion including multiple rip fracture and a small unilateral pneumothorax. Immediate cranial decompression is performed during which she loses approximately 1.5 L of blood. She is admitted to the intensive care unit (ICU) and requires deep sedation and mechanical ventilation after severe brain injury and persistently elevated intracranial pressure. Temperatures rise up to 39.5 Celsius centigrade at day 1 after trauma and external cooling is applied. The patient further receives 1 unit of packed red blood cells as hemoglobin has dropped to 65 g/L (equivalent to 4.0 mmol/L). Within the following 4 hours, oxygenation declines rapidly to an arterial oxygen partial pressure of 60 mmHg with a fraction of inspired oxygen (FiO2) of 1.0. Chest X-ray shows pronounced bilateral infiltrates.
Case 2
An 82-year-old male patient is admitted for hip replacement surgery due to chronic osteoarthritis. He has a history of mild chronic congestive heart failure and type 2 diabetes. The surgery is done in spinal anesthesia. Blood loss is slightly increased as the femoral implant cannot easily be fitted. At day 1 after surgery hemoglobin level is 78 g/L (equivalent to 4.8 mmol/L) and the patient is still tachycardic with a low blood pressure. The patient is given one unit of allogenic packed red blood cells. Tachycardia and blood pressure normalize during the hour of transfusion. However, 3 hours later, the patient develops shortness of breath and fever. Chest radiograph reveals bilateral diffuse infiltrations.
Definition
Defining TRALI is very difficult as the two cases above suggest. Most patient receiving blood products are critically ill and display more than one reason for an acute respiratory distress syndrome (ARDS). Discrimination of possible causes is usually very difficult. In 2004, the Canadian Blood Service (CBS) and Héma-Québec supported a consensus conference on the central questions about TRALI—the most pressing being: how to define TRALI (5)? As there was no evidence through systematic reviews or randomized trials available in the field, the consensus does not contain graded recommendations. However, it was the first attempt to sort the evidence there was about lung injury associated with transfusions (Figure 1). The consensus panel recommends to define TRALI as an acute lung injury (ALI) as defined by the American European Consensus Conference (AECC) on ARDS from 1994 (6), that develops within 6 hours of the transfusion of a blood product, and shall not be related to any other cause of ARDS. ARDS defined by the AECC shows the characteristic acute decline in arterial oxygenation to ratios of partial pressure arterial oxygen and FiO2 (PaO2/FiO2) of below 300 mmHg plus bilateral infiltrates on frontal chest radiograph. The ARDS definition criterion to exclude cardiac or circulatory overload reasons for edema rules out a major differential diagnose associated with transfusion, especially in mass transfusion situations. As to not exclude very mild forms of TRALI, the consensus conference on TRALI allows diagnosis of hypoxemia not only by PaO2/FiO2, but also by oxygen saturations of below 90% when the patient breathes room air. Other risk factors for ARDS are aspiration, pneumonia, toxic inhalation, lung contusion, near drowning, severe sepsis, shock, multiple trauma, burn injury, acute pancreatitis, cardiopulmonary bypass or drug overdose. If any of these conditions coincide with the transfusion of allogenic blood products, the diagnosis “possible TRALI” may be given.
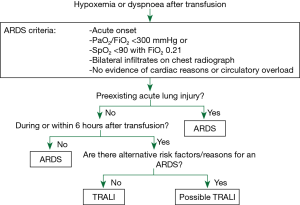
At the same time, published only 3 months later in 2005, another group convened by the US Heart, Lung, and Blood Institute set out to define TRALI. The authors concluded basically on the same criteria, but try to go on further after deciding for “possible TRALI”. The clinical course of patients “with ALI risk factors other than transfusion” should be evaluated for by critical care specialists for a likelihood of TRALI. They may check (I) for “other findings associated with TRALI”; (II) if the patients were stable before transfusion; (III) whether ALI “clearly developed with the transfusion”, and (IV) weigh the likelihood of other risk factors for ALI in this patients against that of an TRALI.
Regarding definition number one by the Canadian Consensus Conference, the patient in case 1 (see above) has a “possible TRALI”, patient in case 2 a “TRALI” by definition.
Regarding the second definition by the US Heart, Lung, and Blood Institute, for the patient in case 1 (see above) TRALI would probably be excluded as a possible diagnosis by a critical care specialist. She has only few additional findings associated with TRALI that developed after the transfusion and the thoracic trauma, invasive ventilation and fever point much stronger towards other reasons for ALI/ARDS. However, laboratory test for TRALI may be performed and give further evidence. The patient in case 2 would be diagnosed a “TRALI” by the second definition.
Several physical and laboratory findings could help to diagnose TRALI. Patients often present with dyspnea, tachypnea, cyanosis, fever, tachycardia, hypotension or froth in the endotracheal tube (7). Typical laboratory findings are transient leukopenia, leukocyte antigen-antibody match between donor and recipient as well as increased neutrophil priming activity (7).
There are a number of strengths and limitations to the definitions. Both use the AECC definition of ALI/ARDS. For many years, this definition has been used frequently and is therefore well known and intensivists are vigilant for such symptoms. However, in 2012 the new Berlin Definition of ARDS was published (8). A revision of the definition of TRALI should be due and the authors might even apply the same methods as the Berlin Group and evaluate their recommendations with the data published since 2005. Another limitation of both definitions is that mild cases of TRALI might get overlooked. Though a low SpO2 at room air as substitute for the PaO2/FiO2 ratio is included, bilateral infiltrates on frontal chest radiograph are still only present in severe cases or lung injury and are an essential part of the AECC definition of ALI/ARDS. The definitions also unwillingly exclude cases with preexisting ARDS that worsen through a transfusion—signifying a double hit. These cases would not even be seen in “possible TRALI”.
The Canadian Consensus Conference gives a very straight forward definition of TRALI that may be applied by any intensivist or even resident in the ICU. TRALI cases with additional risk factors of ALI/ARDS are grouped to “possible TRALI” but are finally reflecting an inhomogeneous group of ALI/ARDS that may or may not be attributable to a transfusion. In contrast, the American Definition of TRALI is much more complex for cases of possible TRALI. A specialist is needed and further testing may be required. Finally, even the working group of this definition states, that there may be cases, which even experts will consider “indeterminate”.
Epidemiology
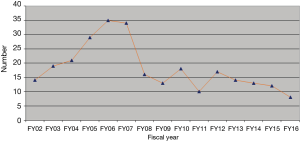
In 2001, the FDA announced TRALI to be the third leading cause of transfusion related death with 13 percent of all transfusion related fatalities (9,10). However, data is from 1976 to 1985 and though the FDA also states TRALI to be a “well-characterized clinical constellation of symptoms”, data from that time show many inconsistencies and differences in diagnosing TRALI. The following years showed, numbers rose to up to 1 case in 4,000 units transfused (11). There are single reports of 1 in 400 transfusion (12). The latest FDA report on fatalities following blood collection and transfusion from 2017 reports TRALI to be the leading reason of transfusion related deaths with 30% from all over the preceding 5 years (13). However, in 2016 there were only 11 cases of TRALI or possible TRALI (18% of total fatalities reported) with transfusion related circulatory overload as the main reason for transfusion related casualties. When looking closely at the data from 2016, only 5 cases were TRALIs, 6 were possible TRALIs. Further, only one of the “real” TRALI cases was classified as “possible” by the FDA as human leucocyte antigen (HLA) matching was done. It seems that measures of restrictive transfusion praxis and TRALI-prevention through, e.g., the reduction of high-volume plasma products have finally led to a significant reduction in TRALI incidence (Figure 2).
Pathophysiology
Pathophysiology of TRALI is not yet fully understood. Additionally, data suggests, that there is no such thing as a single pathomechanism to TRALI. Two general mechanisms to the development of TRALI are agreed upon—leuko-agglutination after leucocyte antibody transfusion with the blood product, and a lipid mediated lung injury from lipids accumulated in blood products after prolonged storage (14).
Leuko-agglutinin theory is based on the mechanistic idea, that after a general activation of granulocytes antibodies through transfusions, granulocytes form tight complexes (leuko-agglutinins) that hinder free transition through small vessels such as lung capillaries. Activation also results in endothelial binding of the leuko-agglutinin. This results in additional endothelial damage and consequently in capillary leakage (15). The integrity of the pulmonary cells is damaged, proteinaceous edema leaks into the alveoli. Antibodies to granulocytes have been found in 89% of the blood products transfused in TRALI cases. Lymphocyte antibodies have been found in 72% of cases (16).
As there is a large group of patients with a TRALI in which no antibodies against immunocompetent cells could be found, the second mechanism by which TRALI may be induced is a two-hit theory independent of antibodies. Here, patients display an underlying disease that increases the general activation level of granulocytes (e.g., sepsis). The lipid containing transfusion acts as a second hit. This theory bases on assumptions made from pathophysiological considerations and animal studies. Lipids and micro-particles accumulate in stored blood products after time (17,18). These lipids and particles are also known to damage endothelial integrity (19,20). One classical disease model of ALI in animals uses oleic acid (21). In a mouse model of ALI, the two-hit approach with an inflammatory first hit, followed by oleic acid as second hit resulted in a severe ARDS (22).
Though both theories are sound, it seems that they are both too mechanistic. New studies imply, that the first mechanism might actually also need a kind of priming or pre-stimulation of neutrophils as proposed for the second mechanism (23). Further, the two-hit model might actually be more likely a “threshold”-model not fixed on the number of hits, but on the combined strength of a multicausal damage and the condition of the patient at the time of transfusion (24).
Patients in both cases could generally by displaying leuko-agglutinins in blood testing. However, patient 1 would be a good example of a multiple hit theory. She has suffered traumatic thorax injury, invasive ventilation and the lungs are further challenged by a multitude of cytotoxic cell debris from the multiple trauma sites. Lipids from the transfusion could easily find a vulnerable endothelial cell barrier.
Blood products
All products containing plasma or plasma components may be a trigger of TRALI. That includes whole blood, apheresis blood, granulocytes, immunoglobulins, etc. Washed red blood cells contain no plasma components and have therefore never been associated with TRALI. However, transfusion of a single unit containing plasma may already be sufficient to trigger a TRALI. It seems that the quantity of HLA class II antibodies, anti-human neutrophil agent and major histocompatibility complex I (MHC-I) monoclonal antibodies in plasma play a major role (11,25). Analyzing for further risks from the donor, female sex clearly correlates with an increased incidence of TRALI in recipients. Especially plasma from female donors that have previously been pregnant seems to be highly suspicious of inducing TRALI. Only very few cases were associated with HLA class I antibodies and with older blood products (11). Recipients of AB plasma have a 14-fold higher risk of developing TRALI than those receiving A, B, or 0 plasma (26).
In 2000/2001, the “Serious Hazard Annual Report” from the UK recommended the exclusion of female donors from plasma products and platelet concentrates (27). Most Blood Services introduced risk-reducing strategies, such as the move to male donors for fresh frozen plasma in 2003, and for suspension of platelet pools, and preferential recruitment of male apheresis platelet donors. Newly recruited female platelet donors are screened for HLA/human neutrophil antigen (HNA) antibodies and retested after pregnancies. With the introduction of these strategies, the number of TRALI cases has decreased from a peak of 36 suspected cases (seven deaths) in 2003 to 11 suspected cases (no deaths) in 2012.
Diagnosis
No specific diagnostic tool exists and as the pathophysiology is very complex, there will probably never be one. General diagnosis is made by clinical appearance and course as described above. However, especially in the case of an immune-mediated TRALI, antibodies might indeed be found and diagnosis confirmed. But considerations may be made carefully as tests are very expensive and time-consuming. A complete case workup consists of both donor and recipients’ plasma to test for HLA class I and II antibodies. As this might also leave the donor with strong feelings of guilt, the Canadian Consensus Panel recommends on a complete case workup with the blood center involved including clinical data and other laboratory findings to rule out other transfusion reactions such as acute hemolytic transfusion reactions (5). If a decision is made for an extensive laboratory testing, the first step requires a testing for HLA class I and II, and HNA-specific antibodies in the donor’s blood. Positive antibody testing should than trigger a specificity testing. Here the antibodies should be specific to an antigen present on the recipient’s white blood cells, or at least a positive reaction between the donors’ serum and the recipients white blood cells should be acquired (positive cross match).
Of all patients reported to the CBS that received mechanical ventilation due to a TRALI 63% had detected leucocyte antibodies, 25% had no antibodies at all. Fifty-seven percent confirmed in cross match donor and recipient (5).
The question of further diagnostic measures is difficult to answer for our above-mentioned cases. In case one, if the definition by the Canadian Consensus Conference is applied, patient would be diagnosed a “possible TRALI”. In that case further diagnostic measures should definitely be taken and a complete workup should be ordered. However, with definition number two by the US Heart, Lung, and Blood Institute, TRALI would probably be excluded as a possible diagnosis by a critical care specialist as other reasons for fever and ALI are more likely. The case of patient number 1 was reported to our blood bank and the blood product was tested for microbiological contamination and increased neutrophil priming activity. All tests were negative. We refrained from a full laboratory workup for TRALI in agreement with blood bank as diagnosis was highly unlikely.
Case 2 would have been diagnosed with a “TRALI” and a full laboratory workup has been ordered. Leucocyte antibodies were confirmed in cross match of donor and recipient likewise.
Treatment
There is no specific therapy for TRALI. Obviously, if there is only the slightest indication that TRALI might be the reason for dyspnea or lung injury, the transfusion should be stopped immediately. The implicated blood product should then be sent to the blood bank for further evaluation regarding a transfusion reaction.
The following clinical management of TRALI is equivalent to that of an ARDS. The foremost concern is adequate oxygenation and decarboxylation. Invasive mechanical ventilation should be avoided if possible, as positive pressure ventilation itself is a risk factor for the development of TRALI (28). If invasive mechanical ventilation is necessary, spontaneous breathing should be allowed. An adequate positive end-expiratory pressure (PEEP) and reduced driving pressure may also reduce further ventilator induced lung injury. Prone positioning may aid the reduction of dorsal atelectasis and improve oxygenation. Intravenous fluid should be reduced to impede further edema of the lungs. Thought pathophysiological considerations for the use of corticosteroids seem sound, current evidence does not support its use in ARDS (29). There are no studies investigating corticosteroids in TRALI apart from isolated case reports.
If supportive care does not result in an improvement of gas exchange and severe hypoxemia seems inevitable, a specialist center for the treatment of ARDS should be consulted and a transfer of the patient considered. Though extracorporeal membrane oxygenation (ECMO) is only very rarely indicated as rescue therapy, patients seem to profit from the transfer into an ECMO center in general (30).
Patient from case 1 was treated with prone positioning while intracranial pressure was monitored tightly. This measure showed a slight but immediate improvement in oxygenation. Percutaneous dilatational tracheotomy was performed on day 3 and sedation ceased. Ventilation management supported spontaneous breathing efforts. On day 8 after the incidence, the patient showed good pulmonary function and had first spontaneous breathing trials without ventilator.
Patient from case 2 was admitted to the ICU and received non-invasive ventilation via face mask. After initial worsening of oxygenation for the first 24 hours, lung function improved quickly and the patient was discharged to the ward at day 3 after onset of TRALI. The donor was informed of the problem and barred from giving blood.
The cases at the beginning of the review demonstrate a common challenge in ICU care. Blood transfusions can be lifesaving yet have potentially serious adverse consequences. Transfusion-related lung injury is one of the most frequent and serious reactions. In addition, recognizing TRALI and distinguish it from a myriad of other causes of hypoxemia and abnormal chest radiograph remains a significant challenge.
Future directions in the field
The most efficient way to reduce the risk for TRALI is to avoid unnecessary transfusion of blood products. Beside the growing evidence in various fields supporting the hypothesis that sparing of blood products may improve outcomes, patient blood management (PBM) programs systematically address this issue and raised awareness for the rational use of blood products especially in the perioperative period. Since the development of TRALI is a multifactorial phenomenon, future treatment strategies should include personalized and patient-tailored approaches. Identification of potential risk factors and pathophysiological pathways in the development of TRALI might help to identify patients at risk for TRALI and moving towards more personalized transfusion regimes.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Plummer NS. Blood Transfusion: A Report of Six Fatalities. Br Med J 1936;2:1186-9. [Crossref] [PubMed]
- Barnard RD. Indiscriminate transfusion: a critique of case reports illustrating hypersensitivity reactions. N Y State J Med 1951;51:2399-402. [PubMed]
- Ward HN. Pulmonary infiltrates associated with leukoagglutinin transfusion reactions. Ann Intern Med 1970;73:689-94. [Crossref] [PubMed]
- Popovsky MA, Abel MD, Moore SB. Transfusion-related acute lung injury associated with passive transfer of antileukocyte antibodies. Am Rev Respir Dis 1983;128:185-9. [Crossref] [PubMed]
- Kleinman S, Caulfield T, Chan P, et al. Toward an understanding of transfusion-related acute lung injury: statement of a consensus panel. Transfusion 2004;44:1774-89. [Crossref] [PubMed]
- Bernard GR, Artigas A, Brigham KL, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994;149:818-24. [Crossref] [PubMed]
- Toy P, Popovsky MA, Abraham E, et al. Transfusion-related acute lung injury: definition and review. Crit Care Med 2005;33:721-6. [Crossref] [PubMed]
- ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 2012;307:2526-33. [PubMed]
- FDA - Food and Drug Administration: Transfusion Related Acute Lung Injury (TRALI). Available online: https://www.fda.gov/biologicsbloodvaccines/safetyavailability/bloodsafety/ucm095556.htm
- Sazama K. Reports of 355 transfusion-associated deaths: 1976 through 1985. Transfusion 1990;30:583-90. [Crossref] [PubMed]
- Toy P, Gajic O, Bacchetti P, et al. Transfusion-related acute lung injury: incidence and risk factors. Blood 2012;119:1757-67. [Crossref] [PubMed]
- Goldman M, Webert KE, Arnold DM, et al. Proceedings of a consensus conference: towards an understanding of TRALI. Transfus Med Rev 2005;19:2-31. [Crossref] [PubMed]
- FDA. Fatalities Reported to FDA Following Blood Collection and Transfusion Annual Summary for FY2016. Available online: https://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/UCM598243.pdf
- Sachs UJ. Pathophysiology of TRALI: current concepts. Intensive Care Med 2007;33 Suppl 1:S3-11. [Crossref] [PubMed]
- Seeger W, Schneider U, Kreusler B, et al. Reproduction of transfusion-related acute lung injury in an ex vivo lung model. Blood 1990;76:1438-44. [PubMed]
- Popovsky MA, Moore SB. Diagnostic and pathogenetic considerations in transfusion-related acute lung injury. Transfusion 1985;25:573-7. [Crossref] [PubMed]
- Silliman CC, Moore EE, Kelher MR, et al. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion 2011;51:2549-54. [Crossref] [PubMed]
- Fu X, Felcyn JR, Odem-Davis K, et al. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: a targeted metabolomics study. Transfusion 2016;56:2560-70. [Crossref] [PubMed]
- Xie RF, Hu P, Wang ZC, et al. Platelet-derived microparticles induce polymorphonuclear leukocyte-mediated damage of human pulmonary microvascular endothelial cells. Transfusion 2015;55:1051-7. [Crossref] [PubMed]
- Silliman CC, Kelher MR, Khan SY, et al. Supernatants and lipids from stored red blood cells activate pulmonary microvascular endothelium through the BLT2 receptor and protein kinase C activation. Transfusion 2017;57:2690-700. [Crossref] [PubMed]
- Matute-Bello G, Frevert CW, Martin TR. Animal models of acute lung injury. Am J Physiol Lung Cell Mol Physiol 2008;295:L379-99. [Crossref] [PubMed]
- Voelker MT, Fichtner F, Kasper M, et al. Characterization of a double-hit murine model of acute respiratory distress syndrome. Clin Exp Pharmacol Physiol 2014;41:844-53. [Crossref] [PubMed]
- Sachs UJ, Hattar K, Weissmann N, et al. Antibody-induced neutrophil activation as a trigger for transfusion-related acute lung injury in an ex vivo rat lung model. Blood 2006;107:1217-9. [Crossref] [PubMed]
- Middelburg RA, van der Bom JG. Transfusion-related acute lung injury not a two-hit, but a multicausal model: TRALI not a Two-Hit, but a Multicausal Model. Transfusion 2015;55:953-60. [Crossref] [PubMed]
- McKenzie CG, Kim M, Singh TK, et al. Peripheral blood monocyte-derived chemokine blockade prevents murine transfusion-related acute lung injury (TRALI). Blood 2014;123:3496-503. [Crossref] [PubMed]
- Eder AF, Dy BA, Perez JM, et al. The residual risk of transfusion-related acute lung injury at the American Red Cross (2008-2011): limitations of a predominantly male-donor plasma mitigation strategy. Transfusion 2013;53:1442-9. [Crossref] [PubMed]
- Bolton-Maggs PH, Cohen H. Serious Hazards of Transfusion (SHOT) haemovigilance and progress is improving transfusion safety. Br J Haematol 2013;163:303-14. [Crossref] [PubMed]
- Rana R, Fernández-Pérez ER, Khan SA, et al. Transfusion-related acute lung injury and pulmonary edema in critically ill patients: a retrospective study. Transfusion 2006;46:1478-83. [Crossref] [PubMed]
- Steinberg KP, Hudson LD, Goodman RB, et al. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N Engl J Med 2006;354:1671-84. [Crossref] [PubMed]
- Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 2009;374:1351-63. [Crossref] [PubMed]


