Advances in lung adenocarcinoma classification: a summary of the new international multidisciplinary classification system (IASLC/ATS/ERS)
Introduction
Lung cancer is the leading cause of cancer death worldwide (1), accounting for 26% of such deaths in women and 28% in men in the USA (2). Of the histologic types of lung cancer, adenocarcinoma is the most common worldwide (3). Recent progress in oncology, radiology, and molecular biology has significantly advanced the understanding of lung adenocarcinoma and its subtypes, which has led to improvements in the paradigm for its clinical management. First, recent progress has highlighted the necessity of distinguishing lung adenocarcinoma from squamous cell carcinoma (SQCC), as several therapies are now available only for adenocarcinoma and certain specific adenocarcinoma mutations (4,5), including: pemetrexed (ineffective in SQCC); bevacizumab (associated with life-threatening hemorrhage in SQCC); crizotinib (targeted to adenocarcinoma with anaplastic lymphoma kinase ALK rearrangements); and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs, first-line therapy for advanced adenocarcinoma with EGFR mutations). Further, recent advances in radiologic-pathologic correlation between computed tomography (CT) and histologic assessments of lung adenocarcinoma have allowed for improved preoperative prediction of its histologic subtype, associated patient prognosis, and multidisciplinary treatment planning. Since the majority of lung cancer patients present at advanced and unresectable stages, the determination of therapy for adenocarcinoma often depends on such radiologic-pathologic correlation and on limited characterization from small biopsy and cytology specimens. Balancing the clinical need for more specific histologic/molecular characterization of adenocarcinoma with the increased use of limited specimens has elevated the level of sophistication in the description and handling of lung adenocarcinoma specimens.
However, the latest World Health Organization (WHO) classification of lung cancer from 2004 preceded these recent advances in the understanding of lung adenocarcinoma and included only limited relevant genetic and clinical criteria into the 2004 classification system. To accommodate these evolving issues, a multidisciplinary consensus of the International Association for the Study of Lung Cancer, American Thoracic Society, and European Respiratory Society (IASLC/ATS/ERS) has sought to accommodate these evolving issues with a new classification system specifically for lung adenocarcinoma, which (I) re-characterizes and expands on certain histologic designations [particularly “mixed” subtype and “bronchioloalveolar carcinoma” (BAC), Table 1]; (II) extrapolates the pathologic classification of resected specimens to a new additional classification system for small biopsy and cytology specimens; and (III) addresses immunohistochemical/molecular, radiologic and surgical considerations. The terminology and criteria of the new classification system are intended to better guide routine patient care and to improve accuracy of data collection for clinical trials (6).

Full table
Resection specimens
In the new IASLC/ATS/ERS system, a distinction is made between atypical adenomatous hyperplasia (AAH), adenocarcinoma in situ (AIS), minimally invasive adenocarcinoma (MIA), invasive adenocarcinoma, and variants of invasive adenocarcinoma (7) (Table 1). The former term BAC, defined as lesions with in situ lepidic growth in the WHO classification, is no longer included in the new system, due to widespread confusion in the clinical and research arenas over its application to a broad range of tumors. The IASLC/ATS/ERS classification instead separates resected specimens formerly called BAC into several new pathologic categories/terms, ranging from preinvasive to invasive lesions (Table 1), associated with varying prognoses. For greater clarity, it also replaces the former “mixed” subtype with predominant histologic subtype characterization of resected specimens.
In the 2004 WHO classification, the preinvasive lesions included only AAH; now, the preinvasive category has expanded to respect a continuum of morphologic changes between AAH and a new category, AIS, one of the lesions formerly called BAC. AAH is a small (usually ≤0.5 cm) proliferation of mild to moderate cell atypia in type II pneumocytes and/or Clara cells. AIS, on the other end of the preinvasive spectrum, is defined as a small neoplasia ≤3.0 cm with pure lepidic growth, along preexisting alveolar structures without stromal, vascular, or pleural invasion, although septal widening with sclerosis is common. AIS is expected to have 100% disease-specific survival when completely resected (8-10).
MIA is another new category of the former BAC lesion and is defined as a predominantly lepidic lesion measuring ≤3.0 cm with only small foci of invasion, the largest of which must be ≤0.5 cm. An invasive component in the new classification system is defined as either cells of a histologic subtype other than lepidic, or invasion of malignant cells into myofibroblastic stroma. For the MIA diagnosis, the tumor cannot contain necrosis or invasion into the lymphatics, blood vessels, or pleura (otherwise, the designation is elevated to invasive adenocarcinoma, detailed below). MIA is expected to have nearly 100% disease-specific survival when completely resected (8-10).
Diagnosis of AIS or MIA requires complete histologic sampling of the tumor and therefore cannot be made from small biopsy/cytology sampling. Most cases of AIS and MIA are nonmucinous but when appropriate should be qualified as mucinous, since preliminary findings suggest that such minimally invasive mucinous lesions will also have high survival rates.
As for a solitary resected adenocarcinoma larger than 3.0 cm that otherwise meets MIA/AIS criteria, without evidence of greater than minimal invasion, there is insufficient evidence regarding survival rate. The suggested designation for such a lesion is “lepidic predominant adenocarcinoma, suspect AIS or MIA”, with a comment stating that an invasive component cannot be excluded if the specimen cannot be completely sampled.
More than 70% to 90% of resected lung adenocarcinomas fall into the invasive adenocarcinoma category. Most invasive adenocarcinomas show heterogeneous histologic patterns, which pathologists traditionally resisted quantifying due to poor reproducibility and instead classified into the “mixed” subtype. This diluted the clinical and prognostic utility of the “mixed” subtype, which relegated it to a wastebasket category and precluded the study of histologic patterns on prognosis. In the new classification system, histologic patterns are described semiquantitatively in 5% increments, and a predominant pattern is deliberately chosen and assigned the largest percentage. This approach may ultimately provide a means for grading lung adenocarcinomas. Intraobserver and interobserver variability appears to be low experienced and specifically trained pathologists perform the histologic assessments (11-15). In addition to prognostication, histologic subtyping is useful in comparing multiple tumors to determine common or different patterns and thus determine if tumors are metastases or separate synchronous or metachronous primaries. This is particularly helpful when a previous tumor’s slides are not available at the time of evaluation of a new tumor.
The major histologic patterns are lepidic, acinar, papillary, micropapillary, and solid. Lepidic-predominant adenocarcinoma (LPA) is nonmucinous and is another of the former BAC lesions. It demonstrates mostly lepidic growth with: (I) at least one focus of invasive adenocarcinoma measuring >0.5 cm; (II) invasion into lymphatics, blood vessels or pleura; or (III) tumor necrosis. Predominant lepidic growth in the invasive adenocarcinomas is associated with favorable prognosis. Micropapillary is also a new major histologic subtype, introduced because such tumors portend a poor prognosis (16,17) similar to that of predominant solid type adenocarcinoma.
Solid-predominant invasive adenocarcinoma must be distinguished from SQCC and large cell carcinoma. Immunohistochemistry (IHC) may be indicated to make this determination in resection specimens. In fact, by utilizing IHC, large cell carcinoma can often be demonstrated to be poorly differentiated adenocarcinoma or SQCC. The prevalence of the diagnosis of large cell carcinoma is expected to diminish over time, if not disappear, as increasingly sensitive and specific markers of cell lineage become available (18,19).
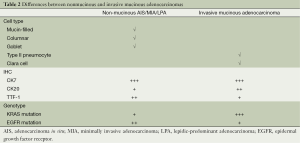
Variants of invasive adenocarcinoma now include, among others (Table 1), the tumors previously classified as mucinous BAC, which in fact usually have usually demonstrated invasive components and are now termed “invasive mucinous adenocarcinoma”. Nonmucinous adenocarcinomas and invasive mucinous adenocarcinoma differ in cell type, in IHC phenotype (Table 2), and in radiologic appearance (further details below). Invasive mucinous adenocarcinoma is also strongly correlated (76%) with KRAS mutation and almost entirely lack EGFR mutation, whereas nonmucinous AIS/MIA/LPA adenocarcinomas demonstrate the converse with 45% positivity for EGFR mutation but only 13% for KRAS mutation (Table 2). Invasive mucinous adenocarcinomas may have the same heterogeneous mixture of lepidic, acinar, papillary, micropapillary, and solid patterns as nonmucinous tumors; however, the clinical significance of semiquantitative histologic pattern reporting for invasive mucinous adenocarcinoma is not yet certain and therefore not included in the new classification system. Invasive mucinous adenocarcinomas show a strong tendency for multicentric, multilobar, and bilateral lung involvement, which may reflect aerogenous spread. Mixtures of mucinous and nonmucinous tumors may rarely occur.
Full table
Colloid adenocarcinoma is an invasive adenocarcinoma variant that now also encompasses the former mucinous cystadenocarcinoma, a very rare lesion that likely is a part of the colloid adenocarcinoma spectrum. A comment can be made to note a resemblance to the former mucinous cystadenocarcinoma.
Enteric adenocarcinoma is added to the classification system to highlight this rare type of lung adenocarcinoma. While it shares morphologic and IHC features with colorectal adenocarcinoma, it is often histologically heterogeneous with some components that resemble primary lung adenocarcinoma (20). Clinical evaluation is needed to exclude a gastrointestinal primary.
Clear cell and signet ring cell features are now known to occur in the setting of various histologic patterns, without evidence of clinical significance beyond a stronger association with the solid pattern (21). Clear cell and signet ring cell features can be recorded if present, but are no longer their own respective subtypes.
Diagnosis on small biopsies/cytology
The histologic heterogeneity of lung adenocarcinoma limits the diagnostic accuracy of small biopsy/cytology specimens when compared to resected specimens. Small biopsy and cytologic specimen also precludes the diagnosis of such lesions as pure in situ adenocarcinoma and/or large cell carcinoma. However, because most lung cancers are not resected, the terminology used for small biopsy and cytology non-small cell lung cancer (NSCLC) diagnoses need to be adequate for treatment. The IASLC/ATS/ERS classification system is the first to provide standardized terminology for diagnosis in small biopsies and cytology (22) (Figure 1).
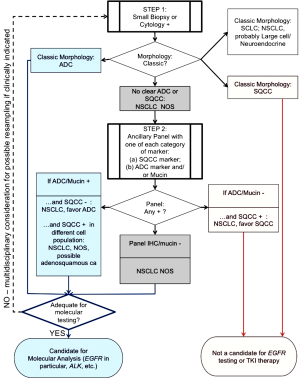
In many cases, adenocarcinoma and SQCC can be distinguished based solely on standard morphologic criteria: glandular architecture for adenocarcinoma and keratinization and intercellular bridges for SQCC. If adenocarcinoma architectural patterns are recognized, they can be mentioned in the report. Cytology also remains a useful adjunct to small biopsies and IHC in reducing diagnostic ambivalence, even proving on occasion to be more informative.
In NSCLC cases where the morphology is not distinctly squamous or adenocarcinoma, an initial histologic/IHC staining panel should be used to refine the diagnosis to “favor adenocarcinoma” or “favor SQCC”, with a comment as to the utilized diagnostic method (light microscopy and/or stain, e.g.,). This limited initial workup helps to preserve the remainder of specimen for any necessary molecular studies or further stains (23-29). Limited staining panels can classify the great majority of primary lung carcinomas using only a single marker each for adenocarcinoma [e.g., thyroid transcription factor-1 (TTF-1), mucin, cytokeratin 7] and SQCC (e.g., p63 in particular, cytokeratin 5/6); additional markers are needed in only a small minority of cases. TTF-1 appears to be the best adenocarcinoma marker (excluding for invasive mucinous adenocarcinoma, where cytokeratin 7 may be more useful), providing added value as a confirmatory pneumocyte marker for primary lung origin, in cases of possible metastatic origin (e.g., colon, breast). Clinico-pathologic correlation can also aid the diagnostic process when considerations include metastases or non-carcinoma primary tumors.
As adenocarcinoma and SQCC markers are generally mutually exclusive, “NSCLC-not otherwise specified (NOS)” can be reserved for cases with strong, concomitant features of both adenocarcinoma and SQCC, using comments to describe the relevant morphologic and/or IHC features. If the IHC features of adenocarcinoma (e.g., TTF-1 positive) and SQCC (e.g., p63 positive) present exclusively from each other in separate cell populations within one sample, the possibility of adenosquamous carcinoma can be suggested, although this diagnosis can be reliably made only with demonstration of 10% of each component on a full resection specimen.
The NSCLC-NOS designation should also be reserved for lung carcinoma cases without any definitive morphologic or IHC features of either adenocarcinoma or SQCC (including cases with only weak/equivocal squamous cell IHC staining). In these cases lacking any differentiating features, a multidisciplinary approach may be the best course, to include discussion of the potential diagnostic benefits and therapeutic impact of other characteristics (e.g., imaging features, clinical phenotype, molecular data, and further sampling) on future management.
It is hoped that the use of IHC will reduce the 20-40% of NSCLCs that would have previously been classified as NSCLC-NOS, to 5% or less of NSCLCs. However, of particular clinical import is that the NSCLC-NOS diagnosis should be strongly considered over NSCLC-favor SQCC in cases of truly equivocal morphology and IHC staining, in order to preserve patient eligibility for further options. A diagnosis of SQCC or NSCLC-favor SQCC currently excludes a patient from histologically driven molecular testing (e.g., EGFR mutation, ALK rearrangement) and related chemotherapy (TKIs, e.g.,), whereas patients with adenocarcinoma, NSCLC-favor adenocarcinoma, or NSCLC-NOS remain candidates for these options. In general, if EGFR mutation is present in an NSCLC-NOS tumor, it is more likely an adenocarcinoma than SQCC.
Because previous clinical trials utilized solely H&E slides for histologic classification, the impact of IHC on classification in future clinical trials needs to be further assessed (6). Limitations of small biopsy/cytology include definitive diagnoses such as large cell carcinoma, adenosquamous carcinoma and pleomorphic carcinoma.
Molecular features/personalized medicine
Histologic molecular correlations for lung adenocarcinoma continue to evolve. The only strong molecular correlation for the predominant histologic subtypes of adenocarcinoma is currently KRAS mutation for invasive mucinous adenocarcinoma (10), which is typically also EGFR mutation negative, with TTF-1 negativity and MUC 2-5-6 positivity due to its origin of bronchiolar mucinous goblet cells. However, several therapies are available only for treatment of adenocarcinoma with certain molecular features (5). The most important current concepts are: (I) TKIs erlotinib and gefitinib are first-line therapy for patients with advanced lung adenocarcinomas with EGFR mutations, which have also been associated with a more indolent course of tumor progression; (II) adenocarcinomas with ALK rearrangements are responsive to crizotinib; (III) pemetrexed is contraindicated in SQCC due to a lack of effectiveness; (IV) SQCC is associated with life-threatening hemorrhage when treated with bevacizumab.
In advanced adenocarcinoma, testing for EGFR mutations and ALK rearrangements is now routine clinical practice. Other molecular targets in adenocarcinoma (for example, ROS1 mutation) and even in SQCC (FGFR1 amplification, DDR2 mutation) will likely also become clinically relevant. Regardless of the target, though further validation is needed, molecular testing offers promise for distinguishing metastases from synchronous primary tumors, even when using small biopsy specimens. Moreover, it may assist in assessing acquired resistance to therapy. For example, EGFR-mutated adenocarcinomas treated with TKIs acquire resistance through EGFR T790M mutation, cMET amplification, dedifferentiation with epithelial-mesenchymal transition, or development of a small cell carcinoma component. For patients with tumor progression after an initial response to targeted therapy, additional biopsies may be indicated to assess for molecular evolution of the tumor.
KRAS mutations are also frequently found in tumors with solid or micropapillary growth. EGFR mutations are most often seen in nonmucinous adenocarcinomas with lepidic, papillary, and possibly micropapillary growth (30-33). For EGFR-mutated tumors, the predominant histologic pattern may predict response to EGFR-TKI therapy (34). ALK rearrangement is associated with acinar growth, cribriform morphology, signet ring cell features, and TTF-1 and p63 co-expression (35,36). Despite imperfect correlation, these histologic-molecular associations emphasize the need for histologic subtyping in clinical practice and for the purposes of clinical trials. Questions surrounding intratumoral heterogeneity of phenotype and genotype require further study.
It is anticipated that molecular testing will be recommended for SQCCs in the near future; molecular targets under investigation include FGFR1 amplifications and DDR2 mutations.
Each institution needs a multidisciplinary strategy for obtaining and processing small biopsy/cytology specimens (37,38). Sufficient tumor must be present for diagnosis and for molecular studies. Most critical molecular studies can be performed using formalin-fixed, paraffin-embedded tissue. Preparation of cell blocks from cytology specimens, including pleural fluids, allows for both IHC and molecular studies. A defined biopsy/cytology protocol established in a multidisciplinary manner provides the best means to tailor lung cancer therapy to individual patients.
Prognostic impact of architectural grading
There is no established grading system for lung adenocarcinoma in resection specimens, and the next revision of TNM staging will likely benefit from consideration of these new classification categories (e.g., AIS, MIA, LPA), as they are unique to adenocarcinoma among the other histologic types of lung cancer and appear to reflect prognosis. Although some studies have addressed architectural grading (39-41), nuclear grading (42-45), or both (46), prognosis for adenocarcinoma is overall probably best predicted by the size of the invasive component, rather than by total tumor size that combines invasive and lepidic components (39,47). Therefore, the best determining factor for T status might be the size of the invasive component, which is addressed by the new classification criteria; for example, AIS might be classified as Tis, and MIA might be classified as Tmi.
Additionally, histologic subtype may help stratify patients; in early stage disease, the lepidic pattern has a favorable prognosis, papillary and acinar patterns have an intermediate prognosis, and a poorer prognosis is associated with micropapillary, mucinous/colloid, and solid patterns (40,48-56). The implications of other patterns (cribriform, fused glands) are also being investigated (13,57,58). Histologic subtyping may eventually help determine which early stage patients should receive adjuvant therapy, as well as who is best suited for limited resection or completion lobectomy (59). Also, its role in distinguishing intrapulmonary metastasis versus synchronous/metachronous primaries holds promise in TNM staging of adenocarcinoma, although a standard surgical algorithm for multiple nodules without mediastinal lymph node invasion has not yet been established. In advanced-stage disease, the prognostic significance of histologic subtyping is unclear (60-63). Further study is needed.
Correlation with imaging
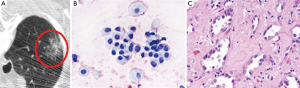
CT can assist treatment planning, particularly when considering sublobar resection, as CT can demonstrate such tumor characteristics as location, appearance and size. CT may be especially useful in cases diagnosed by small biopsy specimens, when pathology alone cannot estimate the tumor size or its degree of total invasion. CT scanning, optimally with thin sections (≤3 mm), can help to further characterize subsolid/semisolid lesions (those containing groundglass and/or a portion of solid component). Similar to the pathologic size cutoff for MIA and AIS, the size threshold for nodule by CT criteria is 3 cm; a larger lesion is radiologically termed a mass. For the spectrum of adenocarcinoma categories, from preinvasive AAH to invasive adenocarcinoma, ground-glass opacity (GGO) on CT scan characteristically corresponds to lepidic tumor growth (Figure 2), while a solid component typically corresponds to invasion (64) (Figure 3), although overlap exists (65-68).
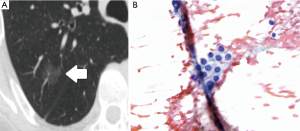
A pure GGO ≤5 mm therefore may represent AAH, while one ≤3 cm in diameter is likely to be AIS (Figure 2). MIA is more variable and has not been fully described on imaging, but in general is expected to contain a small solid component, though predominantly GGO if nonmucinous and more solid if mucinous.
For invasive adenocarcinoma, favorable imaging features include cystic or bubble-like lucencies (Figure 4), intratumoral air bronchogram, extensive ground-glass component, and absence of pleural retraction, which are associated with favorable prognosis, well-differentiated tumor, and/or slow growth in stage IA adenocarcinoma. Thick coarse spiculation, on the other hand, has been associated with lymphovascular involvement and lower post-resection survival. In solid adenocarcinoma, poor outcome and tumor differentiation has been associated with notches/concave cuts within a lesion on imaging.
Invasive mucinous adenocarcinoma, formerly mucinous BAC, possesses a characteristic imaging appearance, often predominantly/entirely solid, with air bronchograms, lobar/multilobar distribution, and potentially multiple scattered opacities (formerly known as multicentric BAC).
Radiology-pathology correlation is also useful in regard to assessment of resection specimens. For lepidic-predominant tumors, multiple tumors, or tumors removed in multiple pieces, gross pathologic examination may misinterpret actual tumor size or number. CT may give a more accurate impression of gross findings and help ensure that each nodule is identified and sampled thoroughly. If there is a discrepancy between CT findings and initial histology, further specimen sampling may be needed for accurate histologic assessment.
Radiology has an expanding role in determination of prognosis, and imaging studies have the potential to influence clinical decision making in new ways. For example, a small GGO (<500 mm3) on CT scan has very little chance of rapid progression and might be considered for close follow-up rather than immediate resection (69). In regard to staging, Murakawa et al. showed that the maximum diameter of a solid tumor component measured in the mediastinal window is a better predictor of survival than the combined solid and ground-glass diameter measured in the lung window (70). This suggests that T status measured by the CT size of the solid component may be more prognostically accurate. Additionally, there is a growing recognition that various histologic features can be predicted by positron emission tomography (PET) (71-76). For example, a high SUVmax is associated with high-grade histology and higher risk of recurrence in stage 1 lung adenocarcinoma (77,78).
Conclusions
Recent advances in the understanding of lung adenocarcinoma have highlighted the clinical significance of histologic subtype, tumor invasion, and immunohistochemical and molecular markers in the prognosis and treatment options for this most common lung cancer type. The new IASLC/ATC/ERS multidisciplinary classification system for this cancer allows for greater diagnostic, clinical and research clarity, by incorporating these advances, addressing small biopsy/cytology specimens, and by eliminating previously confusing terminology. The application of this new classification system will likely improve strategic use of tissue samples and increase diagnostic specificity for clinical and research uses. As it becomes more universally applied, the classification system may provide the groundwork for a dedicated TNM staging criteria for this unique lung cancer type, which will further clarify the paradigm for the best treatment of lung adenocarcinoma.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Castle SM, Salas N, Leveillee RJ. Radio-frequency ablation helps preserve nephrons in salvage of failed microwave ablation for a renal cancer in a solitary kidney. Urol Ann 2013;5:42-4. [PubMed]
- Nakamura H, Saji H. A worldwide trend of increasing primary adenocarcinoma of the lung. Surg Today 2014;44:1004-12. [PubMed]
- Pelosi G, Sonzogni A, Viale G. The classification of lung carcinoma: time to change the morphology-based approach? Int J Surg Pathol 2010;18:161-72. [PubMed]
- Rossi G, Graziano P, Leone A, et al. The role of molecular analyses in the diagnosis and treatment of non-small-cell lung carcinomas. Semin Diagn Pathol 2013;30:298-312. [PubMed]
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol 2013;31:992-1001. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung adenocarcinoma in resected specimens: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:685-705. [PubMed]
- Kadota K, Villena-Vargas J, Yoshizawa A, et al. Prognostic significance of adenocarcinoma in situ, minimally invasive adenocarcinoma, and nonmucinous lepidic predominant invasive adenocarcinoma of the lung in patients with stage I disease. Am J Surg Pathol 2014;38:448-60. [PubMed]
- Yanagawa N, Shiono S, Abiko M, et al. New IASLC/ATS/ERS classification and invasive tumor size are predictive of disease recurrence in stage I lung adenocarcinoma. J Thorac Oncol 2013;8:612-8. [PubMed]
- Yoshizawa A, Sumiyoshi S, Sonobe M, et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: analysis of 440 Japanese patients. J Thorac Oncol 2013;8:52-61. [PubMed]
- Warth A, Stenzinger A, von Brünneck AC, et al. Interobserver variability in the application of the novel IASLC/ATS/ERS classification for pulmonary adenocarcinomas. Eur Respir J 2012;40:1221-7. [PubMed]
- Warth A, Cortis J, Fink L, et al. Training increases concordance in classifying pulmonary adenocarcinomas according to the novel IASLC/ATS/ERS classification. Virchows Arch 2012;461:185-93. [PubMed]
- Wang C, Durra HY, Huang Y, et al. Interobserver reproducibility study of the histological patterns of primary lung adenocarcinoma with emphasis on a more complex glandular pattern distinct from the typical acinar pattern. Int J Surg Pathol 2014;22:149-55. [PubMed]
- Murugan P, Stevenson ME, Hassell LA. Performance validation in anatomic pathology: successful integration of a new classification system into the practice setting using the updated lung non-small cell carcinoma recommendations. Arch Pathol Lab Med 2014;138:105-9. [PubMed]
- Thunnissen E, Beasley MB, Borczuk AC, et al. Reproducibility of histopathological subtypes and invasion in pulmonary adenocarcinoma. An international interobserver study. Mod Pathol 2012;25:1574-83. [PubMed]
- Nitadori J, Bograd AJ, Kadota K, et al. Impact of micropapillary histologic subtype in selecting limited resection vs lobectomy for lung adenocarcinoma of 2cm or smaller. J Natl Cancer Inst 2013;105:1212-20. [PubMed]
- Sumiyoshi S, Yoshizawa A, Sonobe M, et al. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer 2013;81:53-9. [PubMed]
- Rekhtman N, Tafe LJ, Chaft JE, et al. Distinct profile of driver mutations and clinical features in immunomarker-defined subsets of pulmonary large-cell carcinoma. Mod Pathol 2013;26:511-22. [PubMed]
- Rossi G, Mengoli MC, Cavazza A, et al. Large cell carcinoma of the lung: clinically oriented classification integrating immunohistochemistry and molecular biology. Virchows Arch 2014;464:61-8. [PubMed]
- Inamura K, Satoh Y, Okumura S, et al. Pulmonary adenocarcinomas with enteric differentiation: histologic and immunohistochemical characteristics compared with metastatic colorectal cancers and usual pulmonary adenocarcinomas. Am J Surg Pathol 2005;29:660-5. [PubMed]
- Yousem SA. Immunohistochemical and molecular characterization of clear cell carcinoma of the lung. Hum Pathol 2013;44:2467-74. [PubMed]
- Travis WD, Brambilla E, Noguchi M, et al. Diagnosis of lung cancer in small biopsies and cytology: implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch Pathol Lab Med 2013;137:668-84. [PubMed]
- Brunnström H, Johansson L, Jirström K, et al. Immunohistochemistry in the differential diagnostics of primary lung cancer: an investigation within the Southern Swedish Lung Cancer Study. Am J Clin Pathol 2013;140:37-46. [PubMed]
- Montezuma D, Azevedo R, Lopes P, et al. A panel of four immunohistochemical markers (CK7, CK20, TTF-1, and p63) allows accurate diagnosis of primary and metastatic lung carcinoma on biopsy specimens. Virchows Arch 2013;463:749-54. [PubMed]
- Noh S, Shim H. Optimal combination of immunohistochemical markers for subclassification of non-small cell lung carcinomas: A tissue microarray study of poorly differentiated areas. Lung Cancer 2012;76:51-5. [PubMed]
- Rekhtman N, Ang DC, Sima CS, et al. Immunohistochemical algorithm for differentiation of lung adenocarcinoma and squamous cell carcinoma based on large series of whole-tissue sections with validation in small specimens. Mod Pathol 2011;24:1348-59. [PubMed]
- Warth A, Muley T, Herpel E, et al. Large-scale comparative analyses of immunomarkers for diagnostic subtyping of non-small-cell lung cancer biopsies. Histopathology 2012;61:1017-25. [PubMed]
- Kimbrell HZ, Gustafson KS, Huang M, et al. Subclassification of non-small cell lung cancer by cytologic sampling: a logical approach with selective use of immunocytochemistry. Acta Cytol 2012;56:419-24. [PubMed]
- Sterlacci W, Savic S, Schmid T, et al. Tissue-sparing application of the newly proposed IASLC/ATS/ERS classification of adenocarcinoma of the lung shows practical diagnostic and prognostic impact. Am J Clin Pathol 2012;137:946-56. [PubMed]
- Song Z, Zhu H, Guo Z, et al. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med Oncol 2013;30:645. [PubMed]
- Jie-Liu , Li XY, Zhao YQ, et al. Genotype-phenotype correlation in Chinese patients with pulmonary mixed type adenocarcinoma: Relationship between histologic subtypes, TITF-1/SP-A expressions and EGFR mutations. Pathol Res Pract 2014;210:176-81. [PubMed]
- Lee HJ, Kim YT, Kang CH, et al. Epidermal growth factor receptor mutation in lung adenocarcinomas: relationship with CT characteristics and histologic subtypes. Radiology 2013;268:254-64. [PubMed]
- Shim HS. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch Pathol Lab Med 2011;135:1329-34. [PubMed]
- Yoshida T, Ishii G, Goto K, et al. Solid predominant histology predicts EGFR tyrosine kinase inhibitor response in patients with EGFR mutation-positive lung adenocarcinoma. J Cancer Res Clin Oncol 2013;139:1691-700. [PubMed]
- Kim H, Jang SJ, Chung DH, et al. A comprehensive comparative analysis of the histomorphological features of ALK-rearranged lung adenocarcinoma based on driver oncogene mutations: frequent expression of epithelial-mesenchymal transition markers than other genotype. PLoS One 2013;8:e76999. [PubMed]
- Rodig SJ, Mino-Kenudson M, Dacic S, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res 2009;15:5216-23. [PubMed]
- Travis WD, Rekhtman N. Pathological diagnosis and classification of lung cancer in small biopsies and cytology: strategic management of tissue for molecular testing. Semin Respir Crit Care Med 2011;32:22-31. [PubMed]
- Thunnissen E, Kerr KM, Herth FJ, et al. The challenge of NSCLC diagnosis and predictive analysis on small samples. Practical approach of a working group. Lung Cancer 2012;76:1-18. [PubMed]
- Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 2011;24:653-64. [PubMed]
- Sica G, Yoshizawa A, Sima CS, et al. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am J Surg Pathol 2010;34:1155-62. [PubMed]
- Xu L, Tavora F, Burke A. Histologic features associated with metastatic potential in invasive adenocarcinomas of the lung. Am J Surg Pathol 2013;37:1100-8. [PubMed]
- Barletta JA, Yeap BY, Chirieac LR. Prognostic significance of grading in lung adenocarcinoma. Cancer 2010;116:659-69. [PubMed]
- Nakazato Y, Minami Y, Kobayashi H, et al. Nuclear grading of primary pulmonary adenocarcinomas: correlation between nuclear size and prognosis. Cancer 2010;116:2011-9. [PubMed]
- Nakazato Y, Maeshima AM, Ishikawa Y, et al. Interobserver agreement in the nuclear grading of primary pulmonary adenocarcinoma. J Thorac Oncol 2013;8:736-43. [PubMed]
- Petersen I, Kotb WF, Friedrich KH, et al. Core classification of lung cancer: correlating nuclear size and mitoses with ploidy and clinicopathological parameters. Lung Cancer 2009;65:312-8. [PubMed]
- Kadota K, Suzuki K, Kachala SS, et al. A grading system combining architectural features and mitotic count predicts recurrence in stage I lung adenocarcinoma. Mod Pathol 2012;25:1117-27. [PubMed]
- Sawabata N, Kanzaki R, Sakamoto T, et al. Clinical predictor of pre- or minimally invasive pulmonary adenocarcinoma: possibility of sub-classification of clinical T1a. Eur J Cardiothorac Surg 2014;45:256-61. [PubMed]
- Song Z, Zhu H, Guo Z, et al. Prognostic value of the IASLC/ATS/ERS classification in stage I lung adenocarcinoma patients--based on a hospital study in China. Eur J Surg Oncol 2013;39:1262-8. [PubMed]
- Warth A, Muley T, Meister M, et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol 2012;30:1438-46. [PubMed]
- Gu J, Lu C, Guo J, et al. Prognostic significance of the IASLC/ATS/ERS classification in Chinese patients-A single institution retrospective study of 292 lung adenocarcinoma. J Surg Oncol 2013;107:474-80. [PubMed]
- Hu HD, Wan MY, Xu CH, et al. Histological subtypes of solitary pulmonary nodules of adenocarcinoma and their clinical relevance. J Thorac Dis 2013;5:841-6. [PubMed]
- Hung JJ, Jeng WJ, Chou TY, et al. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann Surg 2013;258:1079-86. [PubMed]
- Sakurai H, Asamura H, Miyaoka E, et al. Differences in the prognosis of resected lung adenocarcinoma according to the histological subtype: a retrospective analysis of Japanese lung cancer registry data. Eur J Cardiothorac Surg 2014;45:100-7. [PubMed]
- Tsuta K, Kawago M, Inoue E, et al. The utility of the proposed IASLC/ATS/ERS lung adenocarcinoma subtypes for disease prognosis and correlation of driver gene alterations. Lung Cancer 2013;81:371-6. [PubMed]
- Woo T, Okudela K, Mitsui H, et al. Prognostic value of the IASLC/ATS/ERS classification of lung adenocarcinoma in stage I disease of Japanese cases. Pathol Int 2012;62:785-91. [PubMed]
- Xu L, Tavora F, Battafarano R, et al. Adenocarcinomas with prominent lepidic spread: retrospective review applying new classification of the American Thoracic Society. Am J Surg Pathol 2012;36:273-82. [PubMed]
- Moreira AL, Joubert P, Downey RJ, et al. Cribriform and fused glands are patterns of high-grade pulmonary adenocarcinoma. Hum Pathol 2014;45:213-20. [PubMed]
- Kadota K, Yeh YC, Sima CS, et al. The cribriform pattern identifies a subset of acinar predominant tumors with poor prognosis in patients with stage I lung adenocarcinoma: a conceptual proposal to classify cribriform predominant tumors as a distinct histologic subtype. Mod Pathol 2014;27:690-700. [PubMed]
- Van Schil PE, Asamura H, Rusch VW, et al. Surgical implications of the new IASLC/ATS/ERS adenocarcinoma classification. Eur Respir J 2012;39:478-86. [PubMed]
- Campos-Parra AD, Avilés A, Contreras-Reyes S, et al. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur Respir J 2014;43:1439-47. [PubMed]
- Russell PA, Wainer Z, Wright GM, et al. Does lung adenocarcinoma subtype predict patient survival?: A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society international multidisciplinary lung adenocarcinoma classification. J Thorac Oncol 2011;6:1496-504. [PubMed]
- Russell PA, Barnett SA, Walkiewicz M, et al. Correlation of mutation status and survival with predominant histologic subtype according to the new IASLC/ATS/ERS lung adenocarcinoma classification in stage III (N2) patients. J Thorac Oncol 2013;8:461-8. [PubMed]
- Westaway DD, Toon CW, Farzin M, et al. The International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society grading system has limited prognostic significance in advanced resected pulmonary adenocarcinoma. Pathology 2013;45:553-8. [PubMed]
- Ambrosini-Spaltro A, Ruiu A, Seebacher C, et al. Impact of the IASLC/ATS/ERS classification in pN0 pulmonary adenocarcinomas: a study with radiological-pathological comparisons and survival analyses. Pathol Res Pract 2014;210:40-6. [PubMed]
- Honda T, Kondo T, Murakami S, et al. Radiographic and pathological analysis of small lung adenocarcinoma using the new IASLC classification. Clin Radiol 2013;68:e21-6. [PubMed]
- Lederlin M, Puderbach M, Muley T, et al. Correlation of radio- and histomorphological pattern of pulmonary adenocarcinoma. Eur Respir J 2013;41:943-51. [PubMed]
- Raad RA, Suh J, Harari S, et al. Nodule characterization: subsolid nodules. Radiol Clin North Am 2014;52:47-67. [PubMed]
- Takahashi M, Shigematsu Y, Ohta M, et al. Tumor invasiveness as defined by the newly proposed IASLC/ATS/ERS classification has prognostic significance for pathologic stage IA lung adenocarcinoma and can be predicted by radiologic parameters. J Thorac Cardiovasc Surg 2014;147:54-9. [PubMed]
- van Klaveren RJ, Oudkerk M, Prokop M, et al. Management of lung nodules detected by volume CT scanning. N Engl J Med 2009;361:2221-9. [PubMed]
- Murakawa T, Konoeda C, Ito T, et al. The ground glass opacity component can be eliminated from the T-factor assessment of lung adenocarcinoma. Eur J Cardiothorac Surg 2013;43:925-32. [PubMed]
- Takahashi Y, Takashima S, Watanabe S, et al. F18-FDG PET-CT analyses of small peripheral adenocarcinoma of the lung. Acta Radiol 2013;54:164-8. [PubMed]
- Tanaka R, Nakazato Y, Horikoshi H, et al. Diffusion-weighted imaging and positron emission tomography in various cytological subtypes of primary lung adenocarcinoma. Clin Imaging 2013;37:876-83. [PubMed]
- Chiu CH, Yeh YC, Lin KH, et al. Histological subtypes of lung adenocarcinoma have differential 18F-fluorodeoxyglucose uptakes on the positron emission tomography/computed tomography scan. J Thorac Oncol 2011;6:1697-703. [PubMed]
- Domen H, Hida Y, Okamoto S, et al. Histopathologic characterization of lung adenocarcinoma in relation to fluorine-18-fluorodeoxyglucose uptake on positron emission tomography. Jpn J Clin Oncol 2013;43:874-82. [PubMed]
- Hattori A, Suzuki K, Matsunaga T, et al. Tumour standardized uptake value on positron emission tomography is a novel predictor of adenocarcinoma in situ for c-Stage IA lung cancer patients with a part-solid nodule on thin-section computed tomography scan. Interact Cardiovasc Thorac Surg 2014;18:329-34. [PubMed]
- Lee HY, Jeong JY, Lee KS, et al. Histopathology of lung adenocarcinoma based on new IASLC/ATS/ERS classification: prognostic stratification with functional and metabolic imaging biomarkers. J Magn Reson Imaging 2013;38:905-13. [PubMed]
- Tsutani Y, Miyata Y, Nakayama H, et al. Solid tumor size on high-resolution computed tomography and maximum standardized uptake on positron emission tomography for new clinical T descriptors with T1 lung adenocarcinoma. Ann Oncol 2013;24:2376-81. [PubMed]
- Kadota K, Colovos C, Suzuki K, et al. FDG-PET SUVmax combined with IASLC/ATS/ERS histologic classification improves the prognostic stratification of patients with stage I lung adenocarcinoma. Ann Surg Oncol 2012;19:3598-605. [PubMed]


